Influence of Aerosol Delivered BCG Vaccination on Immunological and Disease Parameters Following SARS-CoV-2 Challenge in Rhesus Macaques
- PMID: 35222355
- PMCID: PMC8863871
- DOI: 10.3389/fimmu.2021.801799
Influence of Aerosol Delivered BCG Vaccination on Immunological and Disease Parameters Following SARS-CoV-2 Challenge in Rhesus Macaques
Abstract
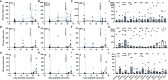
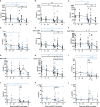
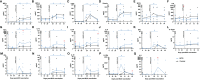
The tuberculosis vaccine, Bacille Calmette-Guerin (BCG), also affords protection against non-tuberculous diseases attributable to heterologous immune mechanisms such as trained innate immunity, activation of non-conventional T-cells, and cross-reactive adaptive immunity. Aerosol vaccine delivery can target immune responses toward the primary site of infection for a respiratory pathogen. Therefore, we hypothesised that aerosol delivery of BCG would enhance cross-protective action against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection and be a deployable intervention against coronavirus disease 2019 (COVID-19). Immune parameters were monitored in vaccinated and unvaccinated rhesus macaques for 28 days following aerosol BCG vaccination. High-dose SARS-CoV-2 challenge was applied by intranasal and intrabronchial instillation and animals culled 6-8 days later for assessment of viral, disease, and immunological parameters. Mycobacteria-specific cell-mediated immune responses were detected following aerosol BCG vaccination, but SARS-CoV-2-specific cellular- and antibody-mediated immunity was only measured following challenge. Early secretion of cytokine and chemokine markers associated with the innate cellular and adaptive antiviral immune response was detected following SARS-CoV-2 challenge in vaccinated animals, at concentrations that exceeded titres measured in unvaccinated macaques. Classical CD14+ monocytes and Vδ2 γδ T-cells quantified by whole-blood immunophenotyping increased rapidly in vaccinated animals following SARS-CoV-2 challenge, indicating a priming of innate immune cells and non-conventional T-cell populations. However, viral RNA quantified in nasal and pharyngeal swabs, bronchoalveolar lavage (BAL), and tissue samples collected at necropsy was equivalent in vaccinated and unvaccinated animals, and in-life CT imaging and histopathology scoring applied to pulmonary tissue sections indicated that the disease induced by SARS-CoV-2 challenge was comparable between vaccinated and unvaccinated groups. Hence, aerosol BCG vaccination did not induce, or enhance the induction of, SARS-CoV-2 cross-reactive adaptive cellular or humoral immunity, although an influence of BCG vaccination on the subsequent immune response to SARS-CoV-2 challenge was apparent in immune signatures indicative of trained innate immune mechanisms and primed unconventional T-cell populations. Nevertheless, aerosol BCG vaccination did not enhance the initial clearance of virus, nor reduce the occurrence of early disease pathology after high dose SARS-CoV-2 challenge. However, the heterologous immune mechanisms primed by BCG vaccination could contribute to the moderation of COVID-19 disease severity in more susceptible species following natural infection.
Keywords: Aerosol BCG vaccination; COVID-19; SARS-CoV-2; cross-protection; macaque; non-specific; trained immunity.
Copyright © 2022 White, Sibley, Sarfas, Morrison, Bewley, Churchward, Fotheringham, Gkolfinos, Gooch, Handley, Humphries, Hunter, Kennard, Longet, Mabbutt, Moffatt, Rayner, Tipton, Watson, Hall, Bodman-Smith, Gleeson, Dennis, Salguero, Carroll, McShane, Cookson, Hopkin and Sharpe.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Protective Efficacy of Inhaled BCG Vaccination Against Ultra-Low Dose Aerosol M. tuberculosis Challenge in Rhesus Macaques.Pharmaceutics. 2020 Apr 25;12(5):394. doi: 10.3390/pharmaceutics12050394. Pharmaceutics. 2020. PMID: 32344890 Free PMC article.
-
BCG vaccination provides protection against IAV but not SARS-CoV-2.Cell Rep. 2022 Mar 8;38(10):110502. doi: 10.1016/j.celrep.2022.110502. Epub 2022 Feb 21. Cell Rep. 2022. PMID: 35235831 Free PMC article.
-
Low-dose M.tb infection but not BCG or MTBVAC vaccination enhances heterologous antibody titres in non-human primates.Front Immunol. 2024 May 10;15:1387454. doi: 10.3389/fimmu.2024.1387454. eCollection 2024. Front Immunol. 2024. PMID: 38799468 Free PMC article.
-
Mitigating Coronavirus Induced Dysfunctional Immunity for At-Risk Populations in COVID-19: Trained Immunity, BCG and "New Old Friends".Front Immunol. 2020 Sep 4;11:2059. doi: 10.3389/fimmu.2020.02059. eCollection 2020. Front Immunol. 2020. PMID: 33013871 Free PMC article. Review.
-
The BCG Vaccine for COVID-19: First Verdict and Future Directions.Front Immunol. 2021 Mar 8;12:632478. doi: 10.3389/fimmu.2021.632478. eCollection 2021. Front Immunol. 2021. PMID: 33763077 Free PMC article. Review.
Cited by
-
Defying convention in the time of COVID-19: Insights into the role of γδ T cells.Front Immunol. 2022 Aug 11;13:819574. doi: 10.3389/fimmu.2022.819574. eCollection 2022. Front Immunol. 2022. PMID: 36032159 Free PMC article. Review.
-
BCG Vaccination: A potential tool against COVID-19 and COVID-19-like Black Swan incidents.Int Immunopharmacol. 2022 Jul;108:108870. doi: 10.1016/j.intimp.2022.108870. Epub 2022 May 17. Int Immunopharmacol. 2022. PMID: 35597119 Free PMC article. Review.
-
Beyond Tuberculosis: The Surprising Immunological Benefits of the Bacillus Calmette-Guérin (BCG) Vaccine in Infectious, Auto-Immune, and Inflammatory Diseases.Pathogens. 2025 Feb 15;14(2):196. doi: 10.3390/pathogens14020196. Pathogens. 2025. PMID: 40005571 Free PMC article. Review.
-
Interplay of Surfactant Protein A and Tumor Necrosis Factor α in Lung and Intestinal Tissues of Rats with Severe Pneumonia.Mol Biotechnol. 2025 Apr 24. doi: 10.1007/s12033-025-01438-0. Online ahead of print. Mol Biotechnol. 2025. PMID: 40272735
-
Immune mechanisms mediating the heterologous effects of BCG vaccination: a systematic review.Front Immunol. 2025 May 19;16:1567111. doi: 10.3389/fimmu.2025.1567111. eCollection 2025. Front Immunol. 2025. PMID: 40458396 Free PMC article.
References
-
- WHO . Coronavirus (COVID-19) Dashboard. Available at: https://covid19.who.int (Accessed September 29, 2021).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

