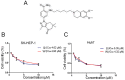
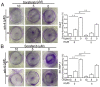
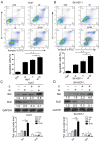
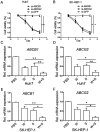
Combination treatment with sorafenib and wh-4 additively suppresses the proliferation of liver cancer cells
- PMID: 35222709
- PMCID: PMC8815050
- DOI: 10.3892/etm.2022.11156
Combination treatment with sorafenib and wh-4 additively suppresses the proliferation of liver cancer cells
Abstract
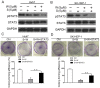
Sorafenib is currently used to treat hepatocellular carcinoma (HCC). However, the development of chemoresistance to sorafenib is a major limitation for sorafenib-based therapy in patients with HCC. In the present study, the effect of the combination therapy of sorafenib and wh-4 on the proliferation of liver cancer cells was investigated. The results showed that sorafenib with wh-4 additively suppressed the proliferation of liver cancer cells. The colony formation of liver cancer cells decreased significantly in response to the combination treatment of sorafenib with wh-4, and it also induced the apoptosis of liver cancer cells. Western blot analysis demonstrated decreased expression of Bcl2, and increased expression of Bax in liver cancer cells treated with a combination of sorafenib and wh-4. Moreover, the migration of liver cancer cells was inhibited. The combination treatment of sorafenib with wh-4 reduced the expression levels of ABCB1 and ABCG2 which are responsible for resistance. Finally, STAT3 overexpression abolished the proliferation inhibition effect of sorafenib with wh-4 on liver cancer cells, and sorafenib and wh-4 suppressed the proliferation of liver cancer cells by STAT3 pathway. Together, these results suggest that sorafenib-wh4 combination treatment is a potential novel therapeutic approach to suppress the proliferation of liver cancer cells.
Keywords: combination treatment; hepatocellular carcinoma cells; proliferation; sorafenib; wh-4.
Copyright: © Chen et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Vitale A, Volk ML, Pastorelli D, Lonardi S, Farinati F, Burra P, Angeli P, Cillo U. Use of sorafenib in patients with hepatocellular carcinoma before liver transplantation: A cost-benefit analysis while awaiting data on sorafenib safety. Hepatology. 2010;51:165–173. doi: 10.1002/hep.23260. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
