Arterial Pulsatility Augments Microcirculatory Perfusion and Maintains the Endothelial Integrity during Extracorporeal Membrane Oxygenation via hsa_circ_0007367 Upregulation in a Canine Model with Cardiac Arrest
- PMID: 35222790
- PMCID: PMC8881135
- DOI: 10.1155/2022/1630918
Arterial Pulsatility Augments Microcirculatory Perfusion and Maintains the Endothelial Integrity during Extracorporeal Membrane Oxygenation via hsa_circ_0007367 Upregulation in a Canine Model with Cardiac Arrest
Abstract
Background: The impairment of microcirculation is associated with the unfavorable outcome for extracorporeal membrane oxygenation (ECMO) patients. Studies revealed that pulsatile modification improves hemodynamics and attenuates inflammation during ECMO support. However, whether flow pattern impacts microcirculation and endothelial integrity is rarely documented. The objective of this work was to explore how pulsatility affects microcirculation during ECMO.
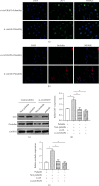
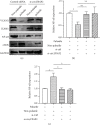
Methods: Canine animal models with cardiac arrest were supported by ECMO, with the i-Cor system used to generate nonpulsatile or pulsatile flow. The sublingual microcirculation parameters were examined using the CytoCam microscope system. The expression of hsa_circ_0007367, a circular RNA, was measured during ECMO support. In vitro validation was performed in pulmonary vascular endothelial cells (PMVECs) exposed to pulsatile or nonpulsatile flow, and the expressions of hsa_circ_0007367, endothelial tight junction markers, endothelial adhesive molecules, endothelial nitric oxide synthases (eNOS), and NF-κB signaling activity were analyzed.
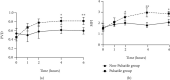
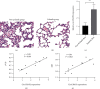
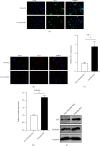
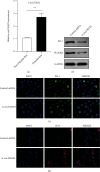
Results: The pulsatile modification of ECMO enhanced microcirculatory perfusion, attenuated pulmonary inflammation, and stabilized endothelial integrity in animal models; meanwhile, the expression of hsa_circ_0007367 was significantly upregulated both in animals and PMVECs exposed to pulsatile flow. In particular, upregulation of hsa_circ_0007367 stabilized the expressions of endothelial tight junction markers zonula occludens- (ZO-) 1 and occludin, followed by modulating the endothelial nitric oxide synthases (eNOS) activity and inhibiting the NF-κB signaling pathway.
Conclusion: The modification of pulsatility contributes to microcirculatory perfusion and endothelial integrity during ECMO. The expression of hsa_circ_0007367 plays a pivotal role in this protective mechanism.
Copyright © 2022 Guanhua Li et al.
Conflict of interest statement
All authors declare no conflict of interest.
Figures
Similar articles
-
Pulsatility protects the endothelial glycocalyx during extracorporeal membrane oxygenation.Microcirculation. 2021 Oct;28(7):e12722. doi: 10.1111/micc.12722. Epub 2021 Jul 16. Microcirculation. 2021. PMID: 34242445
-
Circular RNA UBAP2 (hsa_circ_0007367) Correlates with Microcirculatory Perfusion and Predicts Outcomes of Cardiogenic Shock Patients Undergoing Extracorporeal Membrane Oxygenation Support.Shock. 2022 Jun 1;57(6):200-210. doi: 10.1097/SHK.0000000000001937. Shock. 2022. PMID: 35759302
-
Pulsatile flow increases METTL14-induced m 6 A modification and attenuates septic cardiomyopathy: an experimental study.Int J Surg. 2024 Jul 1;110(7):4103-4115. doi: 10.1097/JS9.0000000000001402. Int J Surg. 2024. PMID: 38549224 Free PMC article.
-
The Disconnect Between Extracorporeal Circulation and the Microcirculation: A Review.ASAIO J. 2022 Jul 1;68(7):881-889. doi: 10.1097/MAT.0000000000001618. Epub 2022 Jan 20. ASAIO J. 2022. PMID: 35067580 Review.
-
Preclinical Studies on Pulsatile Veno-Arterial Extracorporeal Membrane Oxygenation: A Systematic Review.ASAIO J. 2023 May 1;69(5):e167-e180. doi: 10.1097/MAT.0000000000001922. Epub 2023 Mar 27. ASAIO J. 2023. PMID: 36976324
Cited by
-
The mechanisms of glycolipid metabolism disorder on vascular injury in type 2 diabetes.Front Physiol. 2022 Aug 31;13:952445. doi: 10.3389/fphys.2022.952445. eCollection 2022. Front Physiol. 2022. PMID: 36117707 Free PMC article. Review.
-
Circ_0003945: an emerging biomarker and therapeutic target for human diseases.Front Oncol. 2024 Apr 22;14:1275009. doi: 10.3389/fonc.2024.1275009. eCollection 2024. Front Oncol. 2024. PMID: 38711855 Free PMC article. Review.
-
Extracorporeal cardiopulmonary resuscitation: a comparison of two experimental approaches and systematic review of experimental models.Intensive Care Med Exp. 2024 Sep 13;12(1):80. doi: 10.1186/s40635-024-00664-1. Intensive Care Med Exp. 2024. PMID: 39269507 Free PMC article.
-
Optimising fluid therapy during venoarterial extracorporeal membrane oxygenation: current evidence and future directions.Ann Intensive Care. 2025 Mar 19;15(1):32. doi: 10.1186/s13613-025-01458-8. Ann Intensive Care. 2025. PMID: 40106084 Free PMC article. Review.
-
Subtypes and Mechanistic Advances of Extracorporeal Membrane Oxygenation-Related Acute Brain Injury.Brain Sci. 2023 Aug 4;13(8):1165. doi: 10.3390/brainsci13081165. Brain Sci. 2023. PMID: 37626521 Free PMC article. Review.
References
-
- Wollborn J., Siemering S., Steiger C., Buerkle H., Goebel U., Schick M. A. Phosphodiesterase-4 inhibition reduces ECLS-induced vascular permeability and improves microcirculation in a rodent model of extracorporeal resuscitation. American Journal of Physiology Heart and Circulatory Physiology . 2019;316(3):H751–H761. doi: 10.1152/ajpheart.00673.2018. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

