Nrf2 Deficiency Attenuates Testosterone Efficiency in Ameliorating Mitochondrial Function of the Substantia Nigra in Aged Male Mice
- PMID: 35222795
- PMCID: PMC8881137
- DOI: 10.1155/2022/3644318
Nrf2 Deficiency Attenuates Testosterone Efficiency in Ameliorating Mitochondrial Function of the Substantia Nigra in Aged Male Mice
Abstract
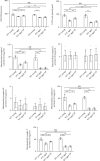
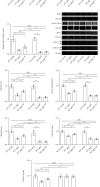
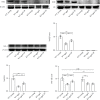
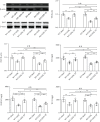
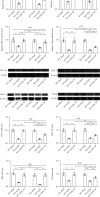
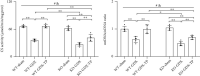
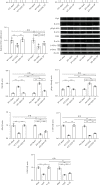
Reduced testosterone level is a common feature of aging in men. Aging, as a risk factor for several neurodegenerative disorders, shows declined mitochondrial function and downregulated mitochondrial biogenesis and mitochondrial dynamics. Mitochondrial biogenesis and mitochondrial dynamics are crucial in maintaining proper mitochondrial function. Supplementation with testosterone is conducive to improving mitochondrial function of males during aging. Nuclear factor erythroid 2-related factor 2 (Nrf2), a regulator of redox homeostasis, is involved in the ameliorative effects of testosterone supplementation upon aging. To explore Nrf2 role in the effects of testosterone supplementation on mitochondrial function during aging, we studied the efficiency of testosterone supplementation in improving mitochondrial function of Nrf2 knockout- (KO-) aged male mice by analyzing the changes of mitochondrial biogenesis and mitochondrial dynamics. It was found that wild-type- (WT-) aged male mice showed low mitochondrial function and expression levels of PGC-1α, NRF-1\NRF-2, and TFAM regulating mitochondrial biogenesis, as well as Drp1, Mfn1, and OPA1 controlling mitochondrial dynamics in the substantia nigra (SN). Nrf2 KO aggravated the defects above in SN of aged male mice. Testosterone supplementation to WT-aged male mice significantly ameliorated mitochondrial function and upregulated mitochondrial biogenesis and mitochondrial dynamics, which were not shown in Nrf2 KO-aged male mice due to Nrf2 deficiency. Testosterone deficiency by gonadectomy (GDX) decreased mitochondrial function, downregulated mitochondrial biogenesis, and altered mitochondrial dynamics balance in young male mice. Supplementation with testosterone to Nrf2 KO-GDX mice only ameliorated the alterations above but did not reverse them to sham level. Nrf2 deficiency attenuated testosterone efficiency in ameliorating mitochondrial function in the SN of aged male mice through mitochondrial biogenesis and mitochondrial dynamics to some extent. Activation of Nrf2 might contribute to testosterone-upregulating mitochondrial biogenesis and mitochondrial dynamics in the SN during aging to produce efficient mitochondria for ATP production.
Copyright © 2022 Baoliang Ren et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures








References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

