Compounds targeting GPI biosynthesis or N-glycosylation are active against Plasmodium falciparum
- PMID: 35222844
- PMCID: PMC8841962
- DOI: 10.1016/j.csbj.2022.01.029
Compounds targeting GPI biosynthesis or N-glycosylation are active against Plasmodium falciparum
Abstract
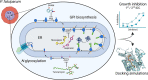
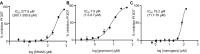
The emergence of resistance to first-line antimalarials, including artemisinin, the last effective malaria therapy in some regions, stresses the urgent need to develop new effective treatments against this disease. The identification and validation of metabolic pathways that could be targeted for drug development may strongly contribute to accelerate this process. In this study, we use fully characterized specific inhibitors targeting glycan biosynthetic pathways as research tools to analyze their effects on the growth of the malaria parasite Plasmodium falciparum and to validate these metabolic routes as feasible chemotherapeutic targets. Through docking simulations using models predicted by AlphaFold, we also shed new light into the modes of action of some of these inhibitors. Molecules inhibiting N-acetylglucosaminyl-phosphatidylinositol de-N-acetylase (GlcNAc-PI de-N-acetylase, PIGL/GPI12) or the inositol acyltransferase (GWT1), central for glycosylphosphatidylinositol (GPI) biosynthesis, halt the growth of intraerythrocytic asexual parasites during the trophozoite stages of the intraerythrocytic developmental cycle (IDC). Remarkably, the nucleoside antibiotic tunicamycin, which targets UDP-N-acetylglucosamine:dolichyl-phosphate N-acetylglucosaminephosphotransferase (ALG7) and N-glycosylation in other organisms, induces a delayed-death effect and inhibits parasite growth during the second IDC after treatment. Our data indicate that tunicamycin induces a specific inhibitory effect, hinting to a more substantial role of the N-glycosylation pathway in P. falciparum intraerythrocytic asexual stages than previously thought. To sum up, our results place GPI biosynthesis and N-glycosylation pathways as metabolic routes with potential to yield much-needed therapeutic targets against the parasite.
Keywords: ALG7, UDP-N-acetylglucosamine:dolichyl-phosphateN-acetylglucosaminephosphotransferase; Antiplasmodial activity; CDS, Coding sequence; CSP, Circumsporozoite protein; DMSO, Dimethyl sulfoxide; ER, Endoplasmic reticulum; GPI, Glycosylphosphatidylinositol; GPI-anchors; GSL-II, Griffonia simplicifoliaII lectin; GWT1, Inositol acyltransferase; GlcNAc, N-acetylglucosamine; GlcNAc-PI de-N-acetylase, N-acetylglucosaminyl-phosphatidylinositol de-N-acetylase; IDC, Intraerythrocytic developmental cycle; Inhibitors; Malaria; N-glycans, Asparagine-linked glycans; N-glycosylation; OST, Oligosaccharyltransferase; Plasmodium falciparum; RBCs, Red blood cells; SHAM, Salicylic hydroxamic acid; UDP-GlcNAc, UDP-N-acetylglucosamine; pLDDT, Predicted local distance difference test.
© 2022 Published by Elsevier B.V. on behalf of Research Network of Computational and Structural Biotechnology.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- WHO | World malaria report 2021. WHO 2021. https://doi.org/ISBN: 978-92-4-004049-6.
-
- WHO | World malaria report 2017. WHO 2018. https://doi.org/ISBN 978-92-4-156552-3.
-
- Wicht KJ, Mok S, Fidock DA. Molecular Mechanisms of Drug Resistance in Plasmodium falciparum Malaria. Https://DoiOrg/101146/Annurev-Micro-020518-115546 2020;74:431–54. https://doi.org/10.1146/ANNUREV-MICRO-020518-115546. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
