An Uncommon Case of Dulaglutide-Related Morbilliform Drug Eruption
- PMID: 35223310
- PMCID: PMC8864188
- DOI: 10.7759/cureus.21536
An Uncommon Case of Dulaglutide-Related Morbilliform Drug Eruption
Abstract
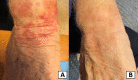
Dulaglutide is a once-weekly injectable glucagon-like peptide-1 (GLP-1) receptor agonist that has shown a durable glycemic efficacy as well as beneficial effects on body weight and major adverse cardiovascular events (MACE) outcomes, making it an important option for the treatment of type 2 diabetes. Common side effects of dulaglutide include nausea, diarrhea, and abdominal distension, and these are usually mild to moderate in severity and tend to diminish over time. Morbilliform drug eruptions to dulaglutide are very rare, with only one case reported until now. We report another case of dulaglutide-morbilliform drug eruption to alert the attending physicians that dulaglutide-related adverse skin reactions should be kept in mind as generalized use of dulaglutide and other GLP-1 receptor agonists are expected to remain in widespread clinical use in the future.
Keywords: cutaneous adverse drug reaction; dulaglutide; glucagon-like peptide-1 receptor agonist; morbilliform drug eruption; skin rash; types 2 diabetes.
Copyright © 2022, Kyriakos et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources