Exploring the Metabolic Landscape of AML: From Haematopoietic Stem Cells to Myeloblasts and Leukaemic Stem Cells
- PMID: 35223487
- PMCID: PMC8867093
- DOI: 10.3389/fonc.2022.807266
Exploring the Metabolic Landscape of AML: From Haematopoietic Stem Cells to Myeloblasts and Leukaemic Stem Cells
Abstract
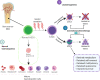
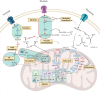
Despite intensive chemotherapy regimens, up to 60% of adults with acute myeloid leukaemia (AML) will relapse and eventually succumb to their disease. Recent studies suggest that leukaemic stem cells (LSCs) drive AML relapse by residing in the bone marrow niche and adapting their metabolic profile. Metabolic adaptation and LSC plasticity are novel hallmarks of leukemogenesis that provide important biological processes required for tumour initiation, progression and therapeutic responses. These findings highlight the importance of targeting metabolic pathways in leukaemia biology which might serve as the Achilles' heel for the treatment of AML relapse. In this review, we highlight the metabolic differences between normal haematopoietic cells, bulk AML cells and LSCs. Specifically, we focus on four major metabolic pathways dysregulated in AML; (i) glycolysis; (ii) mitochondrial metabolism; (iii) amino acid metabolism; and (iv) lipid metabolism. We then outline established and emerging drug interventions that exploit metabolic dependencies of leukaemic cells in the treatment of AML. The metabolic signature of AML cells alters during different biological conditions such as chemotherapy and quiescence. Therefore, targeting the metabolic vulnerabilities of these cells might selectively eradicate them and improve the overall survival of patients with AML.
Keywords: acute myeloid leukaemia; cancer metabolism; leukaemic stem cells; metabolic plasticity; metabolic targeting.
Copyright © 2022 Mesbahi, Trahair, Lock and Connerty.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Acute Myeloid Leukaemia Drives Metabolic Changes in the Bone Marrow Niche.Front Oncol. 2022 Jun 29;12:924567. doi: 10.3389/fonc.2022.924567. eCollection 2022. Front Oncol. 2022. PMID: 35847950 Free PMC article. Review.
-
Transforming the Niche: The Emerging Role of Extracellular Vesicles in Acute Myeloid Leukaemia Progression.Int J Mol Sci. 2024 Apr 17;25(8):4430. doi: 10.3390/ijms25084430. Int J Mol Sci. 2024. PMID: 38674015 Free PMC article. Review.
-
Mitochondrial metabolism as a potential therapeutic target in myeloid leukaemia.Leukemia. 2022 Jan;36(1):1-12. doi: 10.1038/s41375-021-01416-w. Epub 2021 Sep 24. Leukemia. 2022. PMID: 34561557 Free PMC article. Review.
-
Bone Marrow Microenvironment as a Source of New Drug Targets for the Treatment of Acute Myeloid Leukaemia.Int J Mol Sci. 2022 Dec 29;24(1):563. doi: 10.3390/ijms24010563. Int J Mol Sci. 2022. PMID: 36614005 Free PMC article. Review.
-
Cellular automata modelling of leukaemic stem cell dynamics in acute myeloid leukaemia: insights into predictive outcomes and targeted therapies.R Soc Open Sci. 2025 Jan 15;12(1):241202. doi: 10.1098/rsos.241202. eCollection 2025 Jan. R Soc Open Sci. 2025. PMID: 39816742 Free PMC article.
Cited by
-
Leukemic stem cells and advances in hematopoietic stem cell transplantation for acute myeloid leukemia: a narrative review of clinical trials.Stem Cell Investig. 2022 Dec 5;9:10. doi: 10.21037/sci-2022-044. eCollection 2022. Stem Cell Investig. 2022. PMID: 36540355 Free PMC article. Review.
-
Single-cell dissection reveals promotive role of ENO1 in leukemia stem cell self-renewal and chemoresistance in acute myeloid leukemia.Stem Cell Res Ther. 2024 Oct 8;15(1):347. doi: 10.1186/s13287-024-03969-w. Stem Cell Res Ther. 2024. PMID: 39380054 Free PMC article.
-
Crosstalk between DNA methylation and hypoxia in acute myeloid leukaemia.Clin Epigenetics. 2023 Sep 13;15(1):150. doi: 10.1186/s13148-023-01566-x. Clin Epigenetics. 2023. PMID: 37705055 Free PMC article. Review.
-
Unraveling lipid metabolism for acute myeloid leukemia therapy.Curr Opin Hematol. 2025 Mar 1;32(2):77-86. doi: 10.1097/MOH.0000000000000853. Epub 2024 Nov 25. Curr Opin Hematol. 2025. PMID: 39585293 Free PMC article. Review.
-
Resistance to energy metabolism - targeted therapy of AML cells residual in the bone marrow microenvironment.Cancer Drug Resist. 2023 Mar 14;6(1):138-150. doi: 10.20517/cdr.2022.133. eCollection 2023. Cancer Drug Resist. 2023. PMID: 37065866 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

