Aggressive Posterior Retinopathy of Prematurity: Long-Term Outcomes Following Intravitreal Bevacizumab
- PMID: 35223691
- PMCID: PMC8873379
- DOI: 10.3389/fped.2022.778585
Aggressive Posterior Retinopathy of Prematurity: Long-Term Outcomes Following Intravitreal Bevacizumab
Abstract
Purpose: The purpose of this study is to review the neonatal and early childhood course of children who were treated with intravitreal bevacizumab for APROP and identify any long term limitations these children face years after treatment.
Methods: This retrospective consecutive case series reviewed both ophthalmologic and pediatric medical records to determine ocular and neurologic function following treatment with a single injection of intravitreal bevacizumab (IVB) for APROP. Patient records were reviewed to identify the gestational age, average birth weight, gender, post-menstrual age (PMA) at the time of injection, regression status, rescue therapy events, final visual acuity, final refraction, ophthalmologic diagnoses and complications, neurologic diagnoses, and duration of follow up.
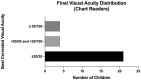
Results: The study included 43 eyes from 13 male and 9 female children. The average gestational age was 24 weeks and average birth weight was 625.2 grams. The average follow-up was 4.08 years (range: 1.85-7.36 years). The average PMA at time of bevacizumab injection was 35.59 weeks. Thirty-five eyes eventually received laser photocoagulation at an average PMA of 53.17 weeks. All eyes in this study demonstrated regression without progression to retinal detachment. At last follow up, 67% (29/43) of eyes were able to discern letters or shapes, with an average visual acuity of 20/37. 16 (72%) children were diagnosed with perinatal neurological disorders. 59% (n = 13) developed chronic neurological impairment, 77% (n = 10) of whom developed neurodevelopmental delay. Several infants were diagnosed with endocrine disease or genetic syndromes.
Conclusions: Extreme prematurity is associated with significant morbidity. Nearly all infants (92%) who developed chronic neurologic disease were diagnosed with neurologic disease during the perinatal period. Intravitreal bevacizumab, often with adjuvant photocoagulation, led to regression without detachment in 100% of eyes, with most verbal children retaining functional vision.
Keywords: APROP; aggressive posterior retinopathy of prematurity; bevicizumab; intravitreal injection; laser; neurodevelopmental outcomes; ophthalmic outcomes; premature (babies).
Copyright © 2022 Naravane, Belin, Rubino and Quiram.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

