Quercetin Administration Following Hypoxia-Induced Neonatal Brain Damage Attenuates Later-Life Seizure Susceptibility and Anxiety-Related Behavior: Modulating Inflammatory Response
- PMID: 35223693
- PMCID: PMC8873174
- DOI: 10.3389/fped.2022.791815
Quercetin Administration Following Hypoxia-Induced Neonatal Brain Damage Attenuates Later-Life Seizure Susceptibility and Anxiety-Related Behavior: Modulating Inflammatory Response
Abstract
Background: Neonatal seizures commonly caused by hypoxia could lead to brain injury and cognitive deficits. Quercetin could cross the blood brain barrier and exerts neuroprotective effects in many neurological disease settings. In this study, we aim to investigate the role of quercetin in attenuating cognitive impairment following hypoxia-induced neonatal seizure (HINS).
Method: Sprague-Dawley rats at P7 were exposed to a premixed gas in a hypoxic chamber to induce brain injury, and then continuously administered with quercetin for 21 days. Pentylenetetrazol kindling was used to induce seizures in the evolution. After the hypoxic lesion was stablished, anxiety-related behavior of rats after HINS was assessed using open field test. Memory impairment of rats after HINS was evaluated using novel object-recognition test and elevated plus maze test. The serum and hippocampal concentrations of TNF-a, iNOS, IL-6 MCP-1, and IL-1β were measured using ELISA. The mRNA expression levels of TNF-a, iNOS, IL-6 in the hippocampus were determined using qRT-PCR. The protein levels of TLR4, NF-κB p65, and p-NF-κB p65 in the hippocampus were determined using Western blot.
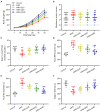
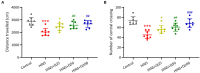
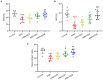
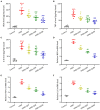
Results: Quercetin administration significantly reduced later-life seizure susceptibility, anxiety-related behavior, and memory impairments in the rats following the HINS when compared to the HINS group without treatment. Both serum and hippocampal proinflammatory cytokines levels were significantly elevated in the rat after HINS. TLR4 protein expressions were increased in the HINS group when compared to control group, and decreased in the group of quercetin. The protein level of p-NF-κB p65 was significantly lower in the quercetin group compared to the HINS group.
Conclusion: We demonstrated that Quercetin significantly reduced susceptibility to later-life seizures. Quercetin could downregulate inflammatory response through TLR4/ NF-κB pathway, thereby attenuating HINS-induced anxiety, hippocampal memory impairment, and cognitive impairment in later life following HINS.
Keywords: NF-κB; TLR4; hypoxia-induced neonatal seizure; inflammation; quercetin.
Copyright © 2022 Wu, Wei, Li, Zhao, Li and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Fingolimod Administration Following Hypoxia Induced Neonatal Seizure Can Restore Impaired Long-term Potentiation and Memory Performance in Adult Rats.Neuroscience. 2023 May 21;519:107-119. doi: 10.1016/j.neuroscience.2023.03.023. Epub 2023 Mar 28. Neuroscience. 2023. PMID: 36990271
-
FTY720 administration following hypoxia-induced neonatal seizure reverse cognitive impairments and severity of seizures in male and female adult rats: The role of inflammation.Neurosci Lett. 2021 Mar 23;748:135675. doi: 10.1016/j.neulet.2021.135675. Epub 2021 Jan 28. Neurosci Lett. 2021. PMID: 33516800
-
The protective effect of DMI on hippocampus EEG, behavioral and biochemical parameters in hypoxia-induced seizure on neonatal period.PLoS One. 2024 Nov 4;19(11):e0309240. doi: 10.1371/journal.pone.0309240. eCollection 2024. PLoS One. 2024. PMID: 39495759 Free PMC article.
-
Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: role of TLR4 in hypoxic microglia.J Neuroinflammation. 2013 Feb 6;10:23. doi: 10.1186/1742-2094-10-23. J Neuroinflammation. 2013. PMID: 23388509 Free PMC article.
-
Quercetin attenuates hypoxia-ischemia induced brain injury in neonatal rats by inhibiting TLR4/NF-κB signaling pathway.Int Immunopharmacol. 2019 Sep;74:105704. doi: 10.1016/j.intimp.2019.105704. Epub 2019 Jun 19. Int Immunopharmacol. 2019. PMID: 31228815
Cited by
-
A Comprehensive Review on Anti-Inflammatory Response of Flavonoids in Experimentally-Induced Epileptic Seizures.Brain Sci. 2023 Jan 5;13(1):102. doi: 10.3390/brainsci13010102. Brain Sci. 2023. PMID: 36672083 Free PMC article. Review.
-
Study on the mechanism of Gastrodiae Rhizoma, Lycii Fructus, and Ziziphi Spinosae Semen in sedation and tranquillising mind.Mol Divers. 2024 Oct;28(5):3279-3294. doi: 10.1007/s11030-023-10756-x. Epub 2023 Nov 2. Mol Divers. 2024. PMID: 37917323
-
Flavonoids as therapeutic agents for epilepsy: unveiling anti-inflammatory and antioxidant pathways for novel treatments.Front Pharmacol. 2024 Sep 12;15:1457284. doi: 10.3389/fphar.2024.1457284. eCollection 2024. Front Pharmacol. 2024. PMID: 39329119 Free PMC article. Review.
-
Inflammatory mediators-induced DNA damage in liver and brain injury: Therapeutic approach of 5-Methoy-N-acetyltryptamine.Toxicol Rep. 2024 Nov 15;13:101816. doi: 10.1016/j.toxrep.2024.101816. eCollection 2024 Dec. Toxicol Rep. 2024. PMID: 39624222 Free PMC article.
-
Overexpression of YKL40,IL-6, IL-8, TNF-α in tonsils and the role of YKL40 in childhood with obstructive sleep apnea syndrome.Sci Rep. 2024 Nov 1;14(1):26283. doi: 10.1038/s41598-024-74402-8. Sci Rep. 2024. PMID: 39487152 Free PMC article.
References
-
- Volpe JJ. Neurology of the newborn. Major Probl Clin Pediatr. (1981) 22:1–648. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

