Transfusion-Free Survival Predicts Severe Retinopathy in Preterm Neonates
- PMID: 35223696
- PMCID: PMC8866869
- DOI: 10.3389/fped.2022.814194
Transfusion-Free Survival Predicts Severe Retinopathy in Preterm Neonates
Abstract
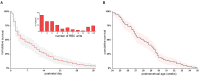
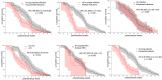
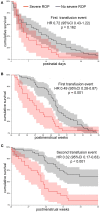
Repeated red blood cell (RBC) transfusions are thought to increase the risk for retinopathy of prematurity (ROP), likely due to a critical fetal hemoglobin (HbF) reduction. In this study, we investigated if the postmenstrual age (PMA) of neonates at transfusion influences the risk for ROP. We estimated the cumulative transfusion-free survival (TFS) in a series of 100 preterm neonates receiving one or more RBC units. TFS was calculated by censoring patients at first transfusion and expressing the time between birth and transfusion as either PMA or postnatal day. Then, we investigated if TFS predicted the occurrence of severe ROP, defined as ROP stage 3 or higher. We found that neonates with severe ROP displayed a significantly shorter TFS expressed according to their PMA (p = 0.001), with similar TFS according to postnatal days. At receiver operating characteristic (ROC) curve analysis, receiving an RBC unit before week 28 of PMA predicted severe ROP with a sensitivity of 64% and a specificity of 78%. In addition, receiving a second RBC unit before the PMA of 29 weeks predicted severe ROP with a sensitivity of 75% and a specificity of 69%. At multivariate analysis, PMA at the second transfusion was even more informative than at first transfusion and outperformed all other variables in predicting severe ROP, with an odds ratio of 4.554 (95% CI 1.332-15.573, p = 0.016). Since HbF decrease is greater after multiple RBC transfusions, it is conceivable that neonates receiving more than one unit before the PMA of 29 weeks may be exposed to a greater disturbance of retinal vascularization. Any strategy aimed at preventing the critical HbF decrease at this low age might potentially reduce the risk for severe ROP.
Keywords: RBC transfusion; fetal hemoglobin; postmenstrual age; preterm birth; retinopathy.
Copyright © 2022 Teofili, Papacci, Bartolo, Molisso, Orlando, Pane, Giannantonio, Serrao, Bianchi, Valentini, Pellegrino, Baldascino, Carducci, Lepore and Vento.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

