Isolation and Investigation of Natural Rare Earth Metal Chelating Agents From Calothrix brevissima - A Step Towards Unraveling the Mechanisms of Metal Biosorption
- PMID: 35223796
- PMCID: PMC8866756
- DOI: 10.3389/fbioe.2022.833122
Isolation and Investigation of Natural Rare Earth Metal Chelating Agents From Calothrix brevissima - A Step Towards Unraveling the Mechanisms of Metal Biosorption
Abstract
In this study water soluble compounds that form complexes with Rare Earth Elements (REE) and other metals were isolated from Calothrix brevissima biomass with chromatographic methods for the first time. Molecular characterization showed that the isolated compounds are most likely polysaccharides comprised of arabinose, xylose, mannose, galactose and glucose. FT-IR analysis revealed functional groups involved in the binding mechanism of Tb are likely sulfate- and to a lesser extend hydroxyl-groups. The binding specificity of the isolated compounds was investigated with different metal solutions. Here, ions of the alkali and alkaline earth metals Na, K, Mg and Ca showed no competition for Tb-binding even at 10-fold excess concentration. Ions of the elements Co and Pb on the other hand replaced Tb at higher concentrations. Addition of the isolated compounds significantly reduced the precipitation of Eu at pH-values between 6.7 and 9.5, indicating that the interaction between the isolated chelators and Rare Earth Metals is stable even at high pH-values.
Keywords: biosorption; calothrix; complexation; cyanobacteria; mechanism; rare earth elements.
Copyright © 2022 Jurkowski, Paper and Brück.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



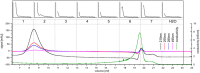
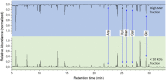
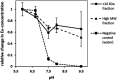

References
-
- Abbas S. H., Ismail I. M., Mostafa T. M., Sulaymon A. H. (2014). Biosorption of Heavy Metals: a Review. J. Chem. Sci. Technol. 3 (4), 74–102.
-
- Al-Kindy S. M. Z., Al-Shamalani K., Suliman F. O., Al-Lawati H. A. J. (2019). Terbium Sensitized Luminescence for the Determination of Fexofenadine in Pharmaceutical Formulations. Arabian J. Chem. 12 (8), 2457–2463. 10.1016/j.arabjc.2015.01.016 - DOI
-
- Bhattacharya P., Mallick K., Ghosh S., Banerjee P., Mukhopadhyay A., Bandyopadhyay S. (2014). Algal Biomass as Potential Biosorbent for Reduction of Organic Load in Gray Water and Subsequent Reuse: Effect on Seed Germination and Enzyme Activity. Bioremediation J. 18 (1), 56–70. 10.1080/10889868.2013.847400 - DOI
LinkOut - more resources
Full Text Sources

