Purification, Characterization, and Hydrolysate Analysis of Dextranase From Arthrobacter oxydans G6-4B
- PMID: 35223821
- PMCID: PMC8867256
- DOI: 10.3389/fbioe.2021.813079
Purification, Characterization, and Hydrolysate Analysis of Dextranase From Arthrobacter oxydans G6-4B
Expression of concern in
-
Expression of Concern: Purification, characterization, and hydrolysate analysis of dextranase from Arthrobacter oxydans G6-4B.Front Bioeng Biotechnol. 2024 Jul 2;12:1450316. doi: 10.3389/fbioe.2024.1450316. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39015139 Free PMC article. No abstract available.
Abstract
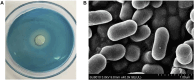
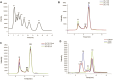
Dextran has aroused increasingly more attention as the primary pollutant in sucrose production and storage. Although enzymatic hydrolysis is more efficient and environmentally friendly than physical methods, the utilization of dextranase in the sugar industry is restricted by the mismatch of reaction conditions and heterogeneity of hydrolysis products. In this research, a dextranase from Arthrobacter oxydans G6-4B was purified and characterized. Through anion exchange chromatography, dextranase was successfully purified up to 32.25-fold with a specific activity of 288.62 U/mg protein and a Mw of 71.12 kDa. The optimum reaction conditions were 55°C and pH 7.5, and it remained relatively stable in the range of pH 7.0-9.0 and below 60°C, while significantly inhibited by metal ions, such as Ni+, Cu2+, Zn2+, Fe3+, and Co2+. Noteworthily, a distinction of previous studies was that the hydrolysates of dextran were basically isomalto-triose (more than 73%) without glucose, and the type of hydrolysates tended to be relatively stable in 30 min; dextranase activity showed a great influence on hydrolysate. In conclusion, given the superior thermal stability and simplicity of hydrolysates, the dextranase in this study presented great potential in the sugar industry to remove dextran and obtain isomalto-triose.
Keywords: Arthrobacter oxydans; dextran; hydrolysis; isomalto-oligosaccharides; purification.
Copyright © 2022 Liu, Li, Dong, Lan, Xu, Wei and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Marine Bacterial Dextranases: Fundamentals and Applications.Molecules. 2022 Aug 28;27(17):5533. doi: 10.3390/molecules27175533. Molecules. 2022. PMID: 36080300 Free PMC article. Review.
-
Characterization of an Alkaline GH49 Dextranase from Marine Bacterium Arthrobacter oxydans KQ11 and Its Application in the Preparation of Isomalto-Oligosaccharide.Mar Drugs. 2019 Aug 19;17(8):479. doi: 10.3390/md17080479. Mar Drugs. 2019. PMID: 31430863 Free PMC article.
-
Purification and characterization of a novel marine Arthrobacter oxydans KQ11 dextranase.Carbohydr Polym. 2014 Jun 15;106:71-6. doi: 10.1016/j.carbpol.2014.01.102. Epub 2014 Feb 8. Carbohydr Polym. 2014. PMID: 24721052
-
Improving stability of a novel dextran-degrading enzyme from marine Arthrobacter oxydans KQ11.Carbohydr Polym. 2014 Mar 15;103:294-9. doi: 10.1016/j.carbpol.2013.12.025. Epub 2013 Dec 24. Carbohydr Polym. 2014. PMID: 24528732
-
Microbial dextran-hydrolyzing enzyme: Properties, structural features, and versatile applications.Food Chem. 2024 Mar 30;437(Pt 2):137951. doi: 10.1016/j.foodchem.2023.137951. Epub 2023 Nov 8. Food Chem. 2024. PMID: 37951078 Review.
Cited by
-
Study of key amino acid residues of GH66 dextranase for producing high-degree polymerized isomaltooligosaccharides and improving of thermostability.Front Bioeng Biotechnol. 2022 Aug 10;10:961776. doi: 10.3389/fbioe.2022.961776. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36032722 Free PMC article.
-
Marine Bacterial Dextranases: Fundamentals and Applications.Molecules. 2022 Aug 28;27(17):5533. doi: 10.3390/molecules27175533. Molecules. 2022. PMID: 36080300 Free PMC article. Review.
-
Purification and characterization of cold-adapted and salt-tolerant dextranase from Cellulosimicrobium sp. THN1 and its potential application for treatment of dental plaque.Front Microbiol. 2022 Nov 11;13:1012957. doi: 10.3389/fmicb.2022.1012957. eCollection 2022. Front Microbiol. 2022. PMID: 36439846 Free PMC article.
References
-
- Abraham K., Weigelt J., Rudolph S., Flöter E. (2019). Systematic Study on the Enzymatic Decomposition of Various Dextran Fractions. Process Biochem. 80, 129–137. 10.1016/j.procbio.2019.01.024 - DOI
-
- Cai R., Lu M., Fang Y., Jiao Y., Zhu Q., Liu Z., et al. (2014). Screening, Production, and Characterization of Dextranase From Catenovulum Sp. Ann. Microbiol. 64, 147–155. 10.1007/s13213-013-0644-7 - DOI
-
- Chew K. W., Chia S. R., Lee S. Y., Zhu L., Show P. L. (2019). Enhanced Microalgal Protein Extraction and Purification Using Sustainable Microwave-Assisted Multiphase Partitioning Technique. Chem. Eng. J. 367, 1–8. 10.1016/j.cej.2019.02.131 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

