Anosmin-1-Like Effect of UMODL1/Olfactorin on the Chemomigration of Mouse GnRH Neurons and Zebrafish Olfactory Axons Development
- PMID: 35223856
- PMCID: PMC8874799
- DOI: 10.3389/fcell.2022.836179
Anosmin-1-Like Effect of UMODL1/Olfactorin on the Chemomigration of Mouse GnRH Neurons and Zebrafish Olfactory Axons Development
Abstract
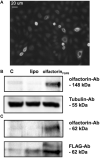
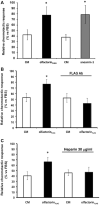
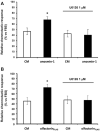
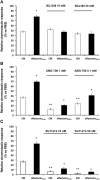
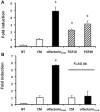
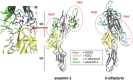
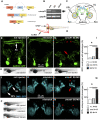
The impairment of development/migration of hypothalamic gonadotropin-releasing hormone (GnRH) neurons is the main cause of Kallmann's syndrome (KS), an inherited disorder characterized by hypogonadism, anosmia, and other developmental defects. Olfactorin is an extracellular matrix protein encoded by the UMODL1 (uromodulin-like 1) gene expressed in the mouse olfactory region along the migratory route of GnRH neurons. It shares a combination of WAP and FNIII repeats, expressed in complementary domains, with anosmin-1, the product of the ANOS1 gene, identified as the causative of KS. In the present study, we have investigated the effects of olfactorin in vitro and in vivo models. The results show that olfactorin exerts an anosmin-1-like strong chemoattractant effect on mouse-immortalized GnRH neurons (GN11 cells) through the activation of the FGFR and MAPK pathways. In silico analysis of olfactorin and anosmin-1 reveals a satisfactory similarity at the N-terminal region for the overall arrangement of corresponding WAP and FNIII domains and marked similarities between WAP domains' binding modes of interaction with the resolved FGFR1-FGF2 complex. Finally, in vivo experiments show that the down-modulation of the zebrafish z-umodl1 gene (orthologous of UMODL1) in both GnRH3:GFP and omp 2k :gap-CFP rw034 transgenic zebrafish strains leads to a clear disorganization and altered fasciculation of the neurites of GnRH3:GFP neurons crossing at the anterior commissure and a significant increase in olfactory CFP + fibers with altered trajectory. Thus, our study shows olfactorin as an additional factor involved in the development of olfactory and GnRH systems and proposes UMODL1 as a gene worthy of diagnostic investigation in KS.
Keywords: GnRH (gonadotropin-releasing hormone); UMODL1; anosmia; anosmin-1; development; hypothalamus and neuroendocrinology; olfactorin; olfactory axons.
Copyright © 2022 Di Schiavi, Vistoli, Moretti, Corrado, Zuccarini, Gervasoni, Casati, Bottai, Merlo and Maggi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The product of X-linked Kallmann's syndrome gene (KAL1) affects the migratory activity of gonadotropin-releasing hormone (GnRH)-producing neurons.Hum Mol Genet. 2004 Nov 15;13(22):2781-91. doi: 10.1093/hmg/ddh309. Epub 2004 Oct 7. Hum Mol Genet. 2004. PMID: 15471890
-
UMODL1/Olfactorin is an extracellular membrane-bound molecule with a restricted spatial expression in olfactory and vomeronasal neurons.Eur J Neurosci. 2005 Jun;21(12):3291-300. doi: 10.1111/j.1460-9568.2005.04164.x. Eur J Neurosci. 2005. PMID: 16026467
-
Anosmin-1 modulates fibroblast growth factor receptor 1 signaling in human gonadotropin-releasing hormone olfactory neuroblasts through a heparan sulfate-dependent mechanism.J Neurosci. 2004 Nov 17;24(46):10384-92. doi: 10.1523/JNEUROSCI.3400-04.2004. J Neurosci. 2004. PMID: 15548653 Free PMC article.
-
X-linked GnRH deficiency: role of KAL-1 mutations in GnRH deficiency.Mol Cell Endocrinol. 2011 Oct 22;346(1-2):13-20. doi: 10.1016/j.mce.2011.04.001. Epub 2011 Apr 8. Mol Cell Endocrinol. 2011. PMID: 21497178 Review.
-
Factors involved in the migration of neuroendocrine hypothalamic neurons.Arch Ital Biol. 2005 Sep;143(3-4):171-8. Arch Ital Biol. 2005. PMID: 16097493 Review.
References
-
- Bassi I., ANDRé V., Marelli F., Vezzoli V., Merlo G. R., Cariboni A., et al. (2016). The Zebrafish: an Emerging Animal Model for Investigating the Hypothalamic Regulation of Reproduction. Minerva Endocrinol. 41, 250–265. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

