Evaluation of the Pink Luminous Breast LED-Based Technology Device as a Screening Tool for the Early Detection of Breast Abnormalities
- PMID: 35223883
- PMCID: PMC8868042
- DOI: 10.3389/fmed.2021.805182
Evaluation of the Pink Luminous Breast LED-Based Technology Device as a Screening Tool for the Early Detection of Breast Abnormalities
Abstract
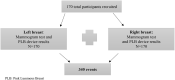
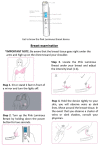
Breast cancer is the leading cause of sex-specific female cancer deaths in the United States. Detection at earlier stages contributes to decreasing the mortality rate. The mammogram is the "Gold Standard" for breast cancer screening with an estimated sensitivity of 86.9% and a specificity of 88.9%. However, these values are negatively affected by the breast density considered a risk factor for developing breast cancer. Herein, we validate the novel LED-based medical device Pink Luminous Breast (PLB) by comparison with the mammogram using a double blinded approach. The PLB works by emitting a LED red light with a harmless spectrum of 640-800 nanometers. This allows the observation of abnormalities represented by dark or shadow areas. In this study, we evaluated the sensitivity and specificity of the PLB device as a screening tool for the early detection of breast abnormalities. Our results show that the PLB device has a high sensitivity (89.6%) and specificity (96.4%) for detecting breast abnormalities comparable to the adjusted mammogram values: 86.3 and 68.9%, respectively. The percentage of presence of breast density was 78.2% using PLB vs. 72.9% with the mammogram. Even with higher findings of breast density, the PLB is still capable of detecting 9.4% of calcifications compared to 6.2% in mammogram results and the reported findings for cysts, masses, or tumor-like abnormalities was higher using the PLB (6.5%) than the mammogram (5.6%). A 100% of the participants felt comfortable using the device without feeling pain or discomfort during the examination with 100% acceptability. The PLB positive validation shows its potential for routine breast screening at non-clinical settings. The PLB provides a rapid, non-invasive, portable, and easy-to-use tool for breast screening that can complement the home-based breast self-examination technique or the clinical breast examination. In addition, the PLB can be conveniently used for screening breasts with surgical implants. PLB provides an accessible and painless breast cancer screening tool. The PLB use is not intended to replace the mammogram for breast screening but rather to use it as an adjunct or complemental tool as part of more efficient earlier detection strategies contributing to decrease mortality rates.
Keywords: LED-based; breast; early-screening; imaging-based diagnoses; mammogram.
Copyright © 2022 Ocasio-Villa, Morales-Torres, Velez-Medina, Cubano, Orengo and Suarez Martinez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- American Cancer Society,. American Cancer Society Recommendations for the Early Detection of Breast Cancer. (2021). Available Online at: https://www.cancer.org/cancer/breast-cancer/screening-tests-and-early-de... (accessed March 17, 2021).
-
- Centers for Disease Control Prevention. U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on 2020 submission data (1999-2018): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. (2021). Available Online at: https://www.cdc.gov/cancer/dataviz (accessed July 20, 2021).
-
- Data Visualizations USCS . Gis.cdc.gov. (2021). Available online at: https://gis.cdc.gov/cancer/uscs/dataviz.html (accessed March 17, 2021).
-
- World Health Organization. Guide to Cancer Early Diagnosis. World Health Organization (2021). Available online at: https://www.who.int/cancer/publications/cancer_early_diagnosis/en/ (accessed March 17, 2021).
LinkOut - more resources
Full Text Sources