Glucocorticoids Inhibit EGFR Signaling Activation in Podocytes in Anti-GBM Crescentic Glomerulonephritis
- PMID: 35223886
- PMCID: PMC8866651
- DOI: 10.3389/fmed.2022.697443
Glucocorticoids Inhibit EGFR Signaling Activation in Podocytes in Anti-GBM Crescentic Glomerulonephritis
Abstract
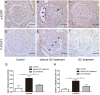
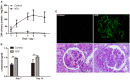
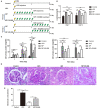
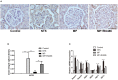
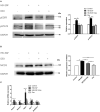
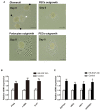
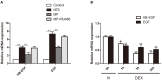
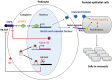
Glucocorticoids are commonly used to treat anti-GBM crescentic glomerulonephritis, however, the mechanism underlying its therapeutic effectiveness is not completely understood. Since podocyte EGFR/STAT3 signaling is known to mediate the development of anti-GBM glomerulonephritis, we investigated the effect of glucocorticoids on EGFR/STAT3 signaling in podocytes. We found that the levels of phosphorylated (activated) EGFR and STAT3 in podocytes were markedly elevated in anti-GBM patients without glucocorticoids treatment, but were normalized in patients with glucocorticoids treatment. In a rat model of anti-GBM glomerulonephritis, glucocorticoids treatment significantly attenuated the proteinuria, crescent formation, parietal epithelial cell (PEC) activation and proliferation, accompanied by elimination of podocyte EGFR/STAT3 signaling activation. In cultured podocytes, glucocorticoids were found to inhibit HB-EGF-induced EGFR and STAT3 activation. The conditioned medium from podocytes treated with HB-EGF in the absence but not presence of glucocorticoids was capable of activating Notch signaling (which is known to be involved in PEC proliferation and crescent formation) and enhancing proliferative activity in primary PECs, suggesting that glucocorticoids prevent podocytes from producing secreted factors that cause PEC proliferation and crescent formation. Furthermore, we found that glucocorticoids can downregulate the expression of EGFR ligands, EGF and HB-EGF, while upregulate the expression of EGFR inhibitor, Gene 33, explaining how glucocorticoids suppress EGFR signaling. Taken together, glucocorticoids exert therapeutic effect on anti-GBM crescentic glomerulonephritis through inhibiting podocyte EGFR/STAT3 signaling and the downstream pathway that leads to PEC proliferation and crescent formation.
Keywords: EGFR/STAT3 signaling; Notch signaling; anti-GBM glomerulonephritis; glucocorticoids; parietal epithelial cell; podocyte.
Copyright © 2022 Wu, Ren, Yang, Song, Tang, Zhang, Zhang, Tang and Shi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Boucher A, Droz D, Adafer E, Noël LH. Relationship between the integrity of Bowman's capsule and the composition of cellular crescents in human crescentic glomerulonephritis. Lab Invest. (1987) 56:526–33. - PubMed
-
- Lan HY, Nikolic-Paterson DJ, Atkins RC. Involvement of activated periglomerular leukocytes in the rupture of Bowman's capsule and glomerular crescent progression in experimental glomerulonephritis. Lab Invest. (1992) 67:743–51. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

