Refining a Protocol for Faecal Microbiota Engraftment in Animal Models After Successful Antibiotic-Induced Gut Decontamination
- PMID: 35223890
- PMCID: PMC8864074
- DOI: 10.3389/fmed.2022.770017
Refining a Protocol for Faecal Microbiota Engraftment in Animal Models After Successful Antibiotic-Induced Gut Decontamination
Abstract
Background: There is mounting evidence for the therapeutic use of faecal microbiota transplant (FMT) in numerous chronic inflammatory diseases. Germ free mice are not always accessible for FMT research and hence alternative approaches using antibiotic depletion prior to FMT in animal studies are often used. Hence, there is a need for standardising gut microbiota depletion and FMT methodologies in animal studies. The aim of this study was to refine gut decontamination protocols prior to FMT engraftment and determine efficiency and stability of FMT engraftment over time.
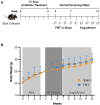
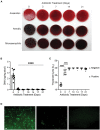
Methods: Male C57BL/6J mice received an antibiotic cocktail consisting of ampicillin, vancomycin, neomycin, and metronidazole in drinking water for 21 days ad libitum. After antibiotic treatment, animals received either FMT or saline by weekly oral gavage for 3 weeks (FMT group or Sham group, respectively), and followed up for a further 5 weeks. At multiple timepoints throughout the model, stool samples were collected and subjected to bacterial culture, qPCR of bacterial DNA, and fluorescent in-situ hybridisation (FISH) to determine bacterial presence and load. Additionally, 16S rRNA sequencing of stool was used to confirm gut decontamination and subsequent FMT engraftment.
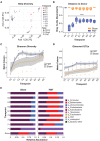
Results: Antibiotic treatment for 7 days was most effective in gut decontamination, as evidenced by absence of bacteria observed in culture, and reduced bacterial concentration, as determined by FISH as well as qPCR. Continued antibiotic administration had no further efficacy on gut decontamination from days 7 to 21. Following gut decontamination, 3 weekly doses of FMT was sufficient for the successful engraftment of donor microbiota in animals. The recolonised animal gut microbiota was similar in composition to the donor sample, and significantly different from the Sham controls as assessed by 16S rRNA sequencing. Importantly, this similarity in composition to the donor sample persisted for 5 weeks following the final FMT dose.
Conclusions: Our results showed that 7 days of broad-spectrum antibiotics in drinking water followed by 3 weekly doses of FMT provides a simple, reliable, and cost-effective methodology for FMT in animal research.
Keywords: antibiotics; faecal microbiota transplant; gut decontamination; gut engraftment; microbiome.
Copyright © 2022 Amorim, McGovern, Raposo, Khatiwada, Shen, Koentgen, Hold, Behary, El-Omar and Zekry.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Comparison of different modes of antibiotic delivery on gut microbiota depletion efficiency and body composition in mouse.BMC Microbiol. 2020 Nov 11;20(1):340. doi: 10.1186/s12866-020-02018-9. BMC Microbiol. 2020. PMID: 33176677 Free PMC article.
-
Minor Effect of Antibiotic Pre-treatment on the Engraftment of Donor Microbiota in Fecal Transplantation in Mice.Front Microbiol. 2019 Nov 21;10:2685. doi: 10.3389/fmicb.2019.02685. eCollection 2019. Front Microbiol. 2019. PMID: 31824463 Free PMC article.
-
Combined Endoscopic and Oral Fecal Microbiota Transplantation in Patients with Antibiotic-Dependent Pouchitis: Low Clinical Efficacy due to Low Donor Microbial Engraftment.Inflamm Intest Dis. 2019 May;4(1):1-6. doi: 10.1159/000497042. Epub 2019 Mar 29. Inflamm Intest Dis. 2019. PMID: 31172007 Free PMC article.
-
Procedures for Fecal Microbiota Transplantation in Murine Microbiome Studies.Front Cell Infect Microbiol. 2021 Sep 21;11:711055. doi: 10.3389/fcimb.2021.711055. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34621688 Free PMC article. Review.
-
Faecal microbiota transplantation for the decolonization of antibiotic-resistant bacteria in the gut: a systematic review and meta-analysis.J Hosp Infect. 2019 Jun;102(2):174-188. doi: 10.1016/j.jhin.2019.03.010. Epub 2019 Mar 26. J Hosp Infect. 2019. PMID: 30926290
Cited by
-
The future of phage therapy in the USA.Trends Mol Med. 2025 Apr 22:S1471-4914(25)00084-X. doi: 10.1016/j.molmed.2025.03.013. Online ahead of print. Trends Mol Med. 2025. PMID: 40268588 Review.
-
Impact of the diet in the gut microbiota after an inter-species microbial transplantation in fish.Sci Rep. 2024 Feb 18;14(1):4007. doi: 10.1038/s41598-024-54519-6. Sci Rep. 2024. PMID: 38369563 Free PMC article.
-
Fecal Microbiota Transplantation Using Donor Stool Obtained from Exercised Mice Suppresses Colonic Tumor Development Induced by Azoxymethane in High-Fat Diet-Induced Obese Mice.Microorganisms. 2025 Apr 27;13(5):1009. doi: 10.3390/microorganisms13051009. Microorganisms. 2025. PMID: 40431182 Free PMC article.
-
Microbial metabolite drives ageing-related clonal haematopoiesis via ALPK1.Nature. 2025 Jun;642(8066):201-211. doi: 10.1038/s41586-025-08938-8. Epub 2025 Apr 23. Nature. 2025. PMID: 40269158 Free PMC article.
-
Interplay of gut microbiota and host epithelial mitochondrial dysfunction is necessary for the development of spontaneous intestinal inflammation in mice.Microbiome. 2023 Nov 17;11(1):256. doi: 10.1186/s40168-023-01686-9. Microbiome. 2023. PMID: 37978573 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

