Mycophenolate Mofetil (CellCept®) in Combination With Low Dose Prednisolone in Moderate to Severe Graves' Orbitopathy
- PMID: 35223896
- PMCID: PMC8873183
- DOI: 10.3389/fmed.2022.788228
Mycophenolate Mofetil (CellCept®) in Combination With Low Dose Prednisolone in Moderate to Severe Graves' Orbitopathy
Abstract
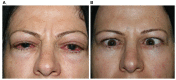
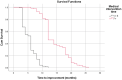
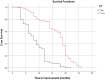
Although corticosteroids are currently the first-choice drug for thyroid eye disease (TED), in 20-30% of cases, patients show poor or non-existent responses, and when the drug is withdrawn, 10-20% of patients relapse. Thus, in this study, we aimed to investigate the efficacy of the combined use of mycophenolate mofetil (CellCept®) and low dose oral prednisolone in patients with moderate to severe Graves' orbitopathy (GO). For the first time, we investigated the relationship between TED-related parameters and proptosis reduction. In a prospective, non-randomized, interventional case series, 242 patients with moderate-to-severe GO were, assigned to receive oral prednisolone (5 mg/ d) and mycophenolate mofetil (CellCept®) (one 500 mg tablet twice per day according to the therapeutic response). The patients were monitored regularly during the 3rd, 6th, 12th, and 18th month of treatment. The main outcome measures were the clinical activity score (CAS), intraocular pressure (IOP), diplopia, proptosis and visual acuity. We also assessed the relationship between the main outcomes with proptosis changes and time to improvement (months). Adverse effects were recorded during each visit. The clinical response rate increased from 67.7% on the third month to 89.2% on the sixth month, and 94.2% on the 12th month. This therapeutic response continued until the 18th month of follow-up. The CAS responses [disease inactivation (CAS <3)] improved during our study: 70.6% on the third month, 90.0% on the sixth month, and 92.5% at 12th month. These conditions continued until the 18th month of follow-up. Proptosis improvement was 52% on the third month, 71% on the sixth month, 83% on the 12th month, and 87.1% on the 18th month. Changes in IOP and visual acuity were not significant (P = 0.568 and 0.668, respectively). The patient showed significant improvement in the Gorman score. A Shorter duration of treatment was seen in patients with earlier onset of intervention, younger age, and lack of all extraocular muscle (EOM) enlargement on computed tomography (CT) scan (p < 0.05). In addition, a better response (more reduction) in proptosis was related to: younger age at disease, earlier treatment intervention (less interval from the time the diagnosis of moderate-to-severe GO was made until medication initiation), shorter treatment time (less time to improvement), less IOP, lack of EOM enlargement on CT scan, and lack of diplopia (P < 0.05). Adverse events occurred in six patients. Findings show that mycophenolate mofetil (CellCept®) plus low-dose prednisolone can be introduced as a new optimal dosing regimen in GO due to its better effect on chronic complications such as proptosis and diplopia.
Keywords: CellCept®; Graves' orbitopathy; mycophenolate mofetil (MMF); prednisolone; thyroid eye disease (TED).
Copyright © 2022 Rajabi, Rafizadeh, Mohammadi, Eshraghi, Mohammadi, Hosseini, Rajabi, Keshmirshekan, Shahriari, Poursayed Lazarjani and Parandin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Efficacy and safety of mycophenolate mofetil in the treatment of moderate to severe Graves' orbitopathy: a meta-analysis.Bioengineered. 2022 Jun;13(6):14719-14729. doi: 10.1080/21655979.2022.2101191. Bioengineered. 2022. PMID: 35959915 Free PMC article. Review.
-
Efficacy and safety of mycophenolate mofetil in patients with active moderate-to-severe Graves' orbitopathy.Clin Endocrinol (Oxf). 2017 Feb;86(2):247-255. doi: 10.1111/cen.13170. Epub 2016 Sep 7. Clin Endocrinol (Oxf). 2017. Retraction in: Clin Endocrinol (Oxf). 2023 May;98(5):743. doi: 10.1111/cen.14888. PMID: 27484048 Retracted. Clinical Trial.
-
Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves' orbitopathy (MINGO): a randomised, observer-masked, multicentre trial.Lancet Diabetes Endocrinol. 2018 Apr;6(4):287-298. doi: 10.1016/S2213-8587(18)30020-2. Epub 2018 Jan 31. Lancet Diabetes Endocrinol. 2018. PMID: 29396246 Clinical Trial.
-
Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials.Lancet Diabetes Endocrinol. 2021 Jun;9(6):360-372. doi: 10.1016/S2213-8587(21)00056-5. Epub 2021 Apr 15. Lancet Diabetes Endocrinol. 2021. PMID: 33865501 Clinical Trial.
-
Systemic safety analysis of mycophenolate in Graves' orbitopathy.J Endocrinol Invest. 2020 Jun;43(6):767-777. doi: 10.1007/s40618-019-01161-z. Epub 2019 Dec 13. J Endocrinol Invest. 2020. PMID: 31834613
Cited by
-
Evaluation of corticoresistance in patients with thyroid eye disease and use of rituximab as a second-line treatment.Endocrine. 2025 Mar;87(3):1112-1119. doi: 10.1007/s12020-024-04108-4. Epub 2024 Nov 28. Endocrine. 2025. PMID: 39604543 Free PMC article.
-
Novel perspectives on the pharmacological treatment of thyroid-associated ophthalmopathy.Front Endocrinol (Lausanne). 2025 Jan 13;15:1469268. doi: 10.3389/fendo.2024.1469268. eCollection 2024. Front Endocrinol (Lausanne). 2025. PMID: 39872310 Free PMC article. Review.
-
Notes for the general paediatrician: managing thyrotoxicosis in children and young people.BMJ Paediatr Open. 2022 Nov;6(1):e001582. doi: 10.1136/bmjpo-2022-001582. BMJ Paediatr Open. 2022. PMID: 36645751 Free PMC article. Review.
-
Management of thyroid eye disease: a Consensus Statement by the American Thyroid Association and the European Thyroid Association.Eur Thyroid J. 2022 Dec 8;11(6):e220189. doi: 10.1530/ETJ-22-0189. Print 2022 Dec 1. Eur Thyroid J. 2022. PMID: 36479875 Free PMC article.
-
Efficacy and safety of mycophenolate mofetil in the treatment of moderate to severe Graves' orbitopathy: a meta-analysis.Bioengineered. 2022 Jun;13(6):14719-14729. doi: 10.1080/21655979.2022.2101191. Bioengineered. 2022. PMID: 35959915 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Medical

