Blood Group Testing
- PMID: 35223922
- PMCID: PMC8873177
- DOI: 10.3389/fmed.2022.827619
Blood Group Testing
Abstract
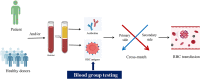
Red blood cell (RBC) transfusion is one of the most frequently performed clinical procedures and therapies to improve tissue oxygen delivery in hospitalized patients worldwide. Generally, the cross-match is the mandatory test in place to meet the clinical needs of RBC transfusion by examining donor-recipient compatibility with antigens and antibodies of blood groups. Blood groups are usually an individual's combination of antigens on the surface of RBCs, typically of the ABO blood group system and the RH blood group system. Accurate and reliable blood group typing is critical before blood transfusion. Serological testing is the routine method for blood group typing based on hemagglutination reactions with RBC antigens against specific antibodies. Nevertheless, emerging technologies for blood group testing may be alternative and supplemental approaches when serological methods cannot determine blood groups. Moreover, some new technologies, such as the evolving applications of blood group genotyping, can precisely identify variant antigens for clinical significance. Therefore, this review mainly presents a clinical overview and perspective of emerging technologies in blood group testing based on the literature. Collectively, this may highlight the most promising strategies and promote blood group typing development to ensure blood transfusion safety.
Keywords: ABO blood group system; RH blood group system; blood group antigen; blood group testing; blood group typing; red blood cell.
Copyright © 2022 Li and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Advances in Blood Typing.Adv Clin Chem. 2016;77:221-269. doi: 10.1016/bs.acc.2016.06.006. Epub 2016 Jul 25. Adv Clin Chem. 2016. PMID: 27717418 Review.
-
Red blood cell alloimmunization and minor red blood cell antigen phenotypes in transfused Ghanaian patients with sickle cell disease.Transfusion. 2019 Jun;59(6):2016-2022. doi: 10.1111/trf.15197. Epub 2019 Feb 13. Transfusion. 2019. PMID: 30758856
-
Blood Group Typing: From Classical Strategies to the Application of Synthetic Antibodies Generated by Molecular Imprinting.Sensors (Basel). 2015 Dec 31;16(1):51. doi: 10.3390/s16010051. Sensors (Basel). 2015. PMID: 26729127 Free PMC article. Review.
-
Optimal blood grouping and antibody screening for safe transfusion.Prilozi. 2009 Jul;30(1):119-28. Prilozi. 2009. PMID: 19736535
-
Red Blood Cell Antigen Genotyping for Sickle Cell Disease, Thalassemia, and Other Transfusion Complications.Transfus Med Rev. 2016 Oct;30(4):197-201. doi: 10.1016/j.tmrv.2016.05.011. Epub 2016 May 28. Transfus Med Rev. 2016. PMID: 27345938 Review.
Cited by
-
ABO Blood Group and the Risk and Prognosis of Lymphoma.J Inflamm Res. 2023 Feb 22;16:769-778. doi: 10.2147/JIR.S401818. eCollection 2023. J Inflamm Res. 2023. PMID: 36855543 Free PMC article. Review.
-
The Discovery of New Antibody in Autoimmune Disease Using a Novel Approach of Coombs Test Based on Flow Cytometry Method.J Clin Lab Anal. 2025 Feb;39(3):e25148. doi: 10.1002/jcla.25148. Epub 2025 Jan 20. J Clin Lab Anal. 2025. PMID: 39829383 Free PMC article.
-
Frequency and distribution of ABO and Rh blood group systems among blood donors at the Northern Zone Blood Transfusion Center in Kilimanjaro, Tanzania: a retrospective cross-sectional study.BMJ Open. 2023 Feb 14;13(2):e068984. doi: 10.1136/bmjopen-2022-068984. BMJ Open. 2023. PMID: 36787973 Free PMC article.
-
The association between blood groups, Rhesus factors, body mass index and obesity among pregnant women at Gadarif Maternity Hospital, Eastern Sudan.BMC Pregnancy Childbirth. 2023 Nov 17;23(1):801. doi: 10.1186/s12884-023-06125-z. BMC Pregnancy Childbirth. 2023. PMID: 37978459 Free PMC article.
-
Prevalence of a Novel Immunogenic Feline Erythrocyte Antigen (FEA 6) and Expression Patterns Between FEAs.J Vet Intern Med. 2025 May-Jun;39(3):e70094. doi: 10.1111/jvim.70094. J Vet Intern Med. 2025. PMID: 40214013 Free PMC article.
References
-
- Yamamoto F. Molecular genetics of ABO. Vox Sang. (2000) 78:91–103. - PubMed
-
- Fung M, Eder A, Spitalnik S, Westhoff C, eds: AABB Technical Manual. 19th ed. Bethesda, MD: AABB Press. (2017)
Publication types
LinkOut - more resources
Full Text Sources

