Engineering hairy cellulose nanocrystals for chemotherapy drug capture
- PMID: 35224320
- PMCID: PMC8865441
- DOI: 10.1016/j.mtchem.2021.100711
Engineering hairy cellulose nanocrystals for chemotherapy drug capture
Abstract
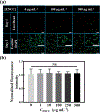
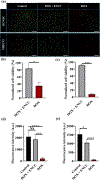
Cancer is one of the leading causes of death worldwide, affecting millions of people every year. While chemotherapy remains one of the most common cancer treatments in the world, the severe side effects of chemotherapy drugs impose serious concerns to cancer patients. In many cases, the chemotherapy can be localized to maximize the drug effects; however, the drug systemic circulation induces undesirable side effects. Here, we have developed a highly efficient cellulose-based nanoadsorbent that can capture more than 6000 mg of doxorubicin (DOX), one of the most widely used chemotherapy drugs, per gram of the adsorbent at physiological conditions. Such drug capture capacity is more than 3200% higher than other nanoadsorbents, such as DNA-based platforms. We show how anionic hairy cellulose nanocrystals, also known as electrosterically stabilized nanocrystalline cellulose (ENCC), bind to positively charged drugs in human serum and capture DOX immediately without imposing any cytotoxicity and hemolytic effects. We elucidate how ENCC provides a remarkable platform for biodetoxification at varying pH, ionic strength, ion type, and protein concentration. The outcome of this research may pave the way for developing the next generation in vitro and in vivo drug capture additives and devices.
Keywords: Cellulose nanocrystal; bioadsorbent; blood purification; chemotherapy; doxorubicin; drug capture; nanocellulose.
Conflict of interest statement
Declaration of interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




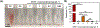
References
-
- Cancer Statistics, Natl. Cancer Inst. (2020). https://www.cancer.gov/about-cancer/understanding/statistics.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
