Substitution of the Methionine Axial Ligand of the T1 Copper for the Fungal-like Phenylalanine Ligand (M298F) Causes Local Structural Perturbations that Lead to Thermal Instability and Reduced Catalytic Efficiency of the Small Laccase from Streptomyces coelicolor A3(2)
- PMID: 35224382
- PMCID: PMC8867573
- DOI: 10.1021/acsomega.1c06668
Substitution of the Methionine Axial Ligand of the T1 Copper for the Fungal-like Phenylalanine Ligand (M298F) Causes Local Structural Perturbations that Lead to Thermal Instability and Reduced Catalytic Efficiency of the Small Laccase from Streptomyces coelicolor A3(2)
Abstract
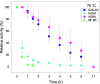
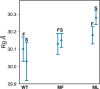
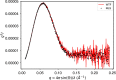
Many industrial processes operate at elevated temperatures or within broad pH and salinity ranges. However, the utilization of enzymes to carry out biocatalysis in such processes is often impractical or even impossible. Laccases (EC 1.10.3.2), which constitute a large family of multicopper oxidases, have long been used in the industrial setting. Although fungal laccases are in many respects considered superior to their bacterial counterparts, the bacterial laccases have been receiving greater attention recently. Albeit lower in redox potential than fungal laccases, bacterial laccases are commonly thermally more stable, act within broader pH ranges, do not contain posttranslational modifications, and could therefore serve as a high potential scaffold for directed evolution for the production of enzymes with enhanced properties. Several examples focusing on the axial ligand mutations of the T1 copper site have been published in the past. However, structural evidence on the local and global changes induced by those mutations have thus far been of computational nature only. In this study, we set out to structurally and kinetically characterize a few of the most commonly reported axial ligand mutations of a bacterial small laccase (SLAC) from Streptomyces coelicolor. While one of the mutations (Met to Leu) equips the enzyme with better thermal stability, the other (Met to Phe) induces an opposite effect. These mutations cause local structural rearrangement of the T1 site as demonstrated by X-ray crystallography. Our analysis confirms past findings that for SLACs, single point mutations that change the identity of the axial ligand of the T1 copper are not enough to provide a substantial increase in the catalytic efficiency but can in some cases have a detrimental effect on the enzyme's thermal stability parameters instead.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
The effect of mutations near the T1 copper site on the biochemical characteristics of the small laccase from Streptomyces coelicolor A3(2).Enzyme Microb Technol. 2015 Jan;68:23-32. doi: 10.1016/j.enzmictec.2014.10.003. Epub 2014 Oct 23. Enzyme Microb Technol. 2015. PMID: 25435502
-
Site-site interactions enhances intramolecular electron transfer in Streptomyces coelicolor laccase.J Am Chem Soc. 2009 Dec 30;131(51):18226-7. doi: 10.1021/ja908793d. J Am Chem Soc. 2009. PMID: 19968274
-
Biochemical studies of the multicopper oxidase (small laccase) from Streptomyces coelicolor using bioactive phytochemicals and site-directed mutagenesis.Microb Biotechnol. 2013 Sep;6(5):588-97. doi: 10.1111/1751-7915.12068. Epub 2013 Jul 1. Microb Biotechnol. 2013. PMID: 23815400 Free PMC article.
-
Insight into stability of CotA laccase from the spore coat of Bacillus subtilis.Biochem Soc Trans. 2007 Dec;35(Pt 6):1579-82. doi: 10.1042/BST0351579. Biochem Soc Trans. 2007. PMID: 18031270 Review.
-
Heterologous laccase production and its role in industrial applications.Bioeng Bugs. 2010 Jul-Aug;1(4):252-62. doi: 10.4161/bbug.1.4.11438. Bioeng Bugs. 2010. PMID: 21327057 Free PMC article. Review.
Cited by
-
Unusual Oligomeric Laccase-like Oxidases from Ascomycete Curvularia geniculata VKM F-3561 Polymerizing Phenylpropanoids and Phenolic Compounds under Neutral Environmental Conditions.Microorganisms. 2023 Nov 3;11(11):2698. doi: 10.3390/microorganisms11112698. Microorganisms. 2023. PMID: 38004710 Free PMC article.
-
Supramolecular enzyme-mimicking catalysts self-assembled from peptides.iScience. 2022 Dec 20;26(1):105831. doi: 10.1016/j.isci.2022.105831. eCollection 2023 Jan 20. iScience. 2022. PMID: 36636357 Free PMC article. Review.
-
Unexpected effect of an axial ligand mutation in the type 1 copper center in small laccase: structure-based analyses and engineering to increase reduction potential and activity.Chem Sci. 2025 May 19;16(25):11339-11346. doi: 10.1039/d5sc02177d. eCollection 2025 Jun 25. Chem Sci. 2025. PMID: 40438172 Free PMC article.
References
-
- Dwivedi U. N.; Singh P.; Pandey V. P.; Kumar A. Structure-function relationship among bacterial, fungal and plant laccases. J. Mol. Catal. B Enzym. 2011, 68, 117–128. 10.1016/j.molcatb.2010.11.002. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

