Biotechnological synthesis of Pd-based nanoparticle catalysts
- PMID: 35224444
- PMCID: PMC8805459
- DOI: 10.1039/d1na00686j
Biotechnological synthesis of Pd-based nanoparticle catalysts
Abstract
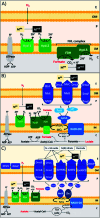
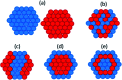
Palladium metal nanoparticles are excellent catalysts used industrially for reactions such as hydrogenation and Heck and Suzuki C-C coupling reactions. However, the global demand for Pd far exceeds global supply, therefore the sustainable use and recycling of Pd is vital. Conventional chemical synthesis routes of Pd metal nanoparticles do not meet sustainability targets due to the use of toxic chemicals, such as organic solvents and capping agents. Microbes are capable of bioreducing soluble high oxidation state metal ions to form metal nanoparticles at ambient temperature and pressure, without the need for toxic chemicals. Microbes can also reduce metal from waste solutions, revalorising these waste streams and allowing the reuse of precious metals. Pd nanoparticles supported on microbial cells (bio-Pd) can catalyse a wide array of reactions, even outperforming commercial heterogeneous Pd catalysts in several studies. However, to be considered a viable commercial option, the intrinsic activity and selectivity of bio-Pd must be enhanced. Many types of microorganisms can produce bio-Pd, although most studies so far have been performed using bacteria, with metal reduction mediated by hydrogenase or formate dehydrogenase enzymes. Dissimilatory metal-reducing bacteria (DMRB) possess additional enzymes adapted for extracellular electron transport that potentially offer greater control over the properties of the nanoparticles produced. A recent and important addition to the field are bio-bimetallic nanoparticles, which significantly enhance the catalytic properties of bio-Pd. In addition, systems biology can integrate bio-Pd into biocatalytic processes, and processing techniques may enhance the catalytic properties further, such as incorporating additional functional nanomaterials. This review aims to highlight aspects of enzymatic metal reduction processes that can be bioengineered to control the size, shape, and cellular location of bio-Pd in order to optimise its catalytic properties.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

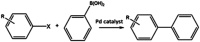




References
-
- Malleron J.-L., Fiaud J.-C. and Legros J.-Y., WACKER Process, in Handbook of Palladium-Catalyzed Organic Reactions, 1997
-
- Harrowven D. C., Handbook of Organopalladium Chemistry for Organic Synthesis, Stuttg, 2003
-
- Liu X. Astruc D. Development of the Applications of Palladium on Charcoal in Organic Synthesis. Adv. Synth. Catal. 2018;360:3426–3459. doi: 10.1002/adsc.201800343. - DOI
-
- Torborg C. Beller M. Recent applications of palladium-catalyzed coupling reactions in the pharmaceutical, agrochemical, and fine chemical industries. Adv. Synth. Catal. 2009;351(18):3022–3043. doi: 10.1002/adsc.200900587. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

