No substantial preexisting B cell immunity against SARS-CoV-2 in healthy adults
- PMID: 35224466
- PMCID: PMC8857777
- DOI: 10.1016/j.isci.2022.103951
No substantial preexisting B cell immunity against SARS-CoV-2 in healthy adults
Abstract
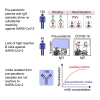
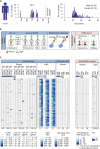
Preexisting immunity against SARS-CoV-2 may have critical implications for our understanding of COVID-19 susceptibility and severity. The presence and clinical relevance of a preexisting B cell immunity remain to be fully elucidated. Here, we provide a detailed analysis of the B cell immunity to SARS-CoV-2 in unexposed individuals. To this end, we extensively investigated SARS-CoV-2 humoral immunity in 150 adults sampled pre-pandemically. Comprehensive screening of donor plasma and purified IgG samples for binding and neutralization in various functional assays revealed no substantial activity against SARS-CoV-2 but broad reactivity to endemic betacoronaviruses. Moreover, we analyzed antibody sequences of 8,174 putatively SARS-CoV-2-reactive B cells at a single cell level and generated and tested 158 monoclonal antibodies. None of these antibodies displayed relevant binding or neutralizing activity against SARS-CoV-2. Taken together, our results show no evidence of competent preexisting antibody and B cell immunity against SARS-CoV-2 in unexposed adults.
Keywords: Immune response; Immunity; Virology.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Arvin A.M., Fink K., Schmid M.A., Cathcart A., Spreafico R., Havenar-Daughton C., Lanzavecchia A., Corti D., Virgin H.W. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. 2020;584:353–363. - PubMed
-
- Barnes C.O., West A.P., Huey-Tubman K.E., Hoffmann M.A.G., Sharaf N.G., Hoffman P.R., Koranda N., Gristick H.B., Gaebler C., Muecksch F., et al. Structures of human antibodies bound to SARS-CoV-2 spike reveal common epitopes and recurrent features of antibodies. Cell. 2020;182:828–842.e16. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

