Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study
- PMID: 35224524
- PMCID: PMC8863504
- DOI: 10.1016/S2666-7568(22)00012-5
Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study
Abstract
Background: The use of COVID-19 vaccines has been prioritised to protect the most vulnerable-notably, older people. Because of fluctuations in vaccine availability, strategies such as delayed second dose and heterologous prime-boost have been used. However, the effectiveness of these strategies in frail, older people are unknown. We aimed to assess the antigenicity of mRNA-based COVID-19 vaccines in frail, older people in a real-world setting, with a rationed interval dosing of 16 weeks between the prime and boost doses.
Methods: This prospective observational cohort study was done across 12 long-term care facilities of the Montréal Centre-Sud - Integrated University Health and Social Services Centre in Montréal, Québec, Canada. Under a rationing strategy mandated by the provincial government, adults aged 65 years and older residing in long-term care facilities in Québec, Canada, with or without previously documented SARS-CoV-2 infection, were administered homologous or heterologous mRNA vaccines, with an extended 16-week interval between doses. All older residents in participating long-term care facilities who received two vaccine doses were eligible for inclusion in this study. Participants were enrolled from Dec 31, 2020, to Feb 16, 2021, and data were collected up to June 9, 2021. Clinical data and blood samples were serially collected from participants at the following timepoints: at baseline, before the first dose; 4 weeks after the first dose; 6-10 weeks after the first dose; 16 weeks after the first dose, up to 2 days before administration of the second dose; and 4 weeks after the second dose. Sera were tested for SARS-CoV-2-specific IgG antibodies (to the trimeric spike protein, the receptor-binding domain [RBD] of the spike protein, and the nucleocapsid protein) by automated chemiluminescent ELISA. Two cohorts were used in this study: a discovery cohort, for which blood samples were collected before administration of the first vaccine dose and longitudinally thereafter; and a confirmatory cohort, for which blood samples were only collected from 4 weeks after the prime dose. Analyses were done in the discovery cohort, with validation in the confirmatory cohort, when applicable.
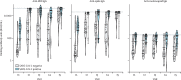
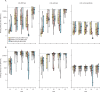
Findings: The total study sample consisted of 185 participants. 65 participants received two doses of mRNA-1273 (Spikevax; Moderna), 36 received two doses of BNT162b2 (Comirnaty; Pfizer-BioNTech), and 84 received mRNA-1273 followed by BNT162b2. In the discovery cohort, after a significant increase in anti-RBD and anti-spike IgG concentrations 4 weeks after the prime dose (from 4·86 log binding antibody units [BAU]/mL to 8·53 log BAU/mL for anti-RBD IgG and from 5·21 log BAU/mL to 8·05 log BAU/mL for anti-spike IgG), there was a significant decline in anti-RBD and anti-spike IgG concentrations until the boost dose (7·10 log BAU/mL for anti-RBD IgG and 7·60 log BAU/mL for anti-spike IgG), followed by an increase 4 weeks later for both vaccines (9·58 log BAU/mL for anti-RBD IgG and 9·23 log BAU/mL for anti-spike IgG). SARS-CoV-2-naive individuals showed lower antibody responses than previously infected individuals at all timepoints tested up to 16 weeks after the prime dose, but achieved similar antibody responses to previously infected participants by 4 weeks after the second dose. Individuals primed with the BNT162b2 vaccine showed a larger decrease in mean anti-RBD and anti-spike IgG concentrations with a 16-week interval between doses (from 8·12 log BAU/mL to 4·25 log BAU/mL for anti-RBD IgG responses and from 8·18 log BAU/mL to 6·66 log BAU/mL for anti-spike IgG responses) than did those who received the mRNA-1273 vaccine (two doses of mRNA-1273: from 8·06 log BAU/mL to 7·49 log BAU/mL for anti-RBD IgG responses and from 6·82 log BAU/mL to 7·56 log BAU/mL for anti-spike IgG responses; mRNA-1273 followed by BNT162b2: from 8·83 log BAU/mL to 7·95 log BAU/mL for anti-RBD IgG responses and from 8·50 log BAU/mL to 7·97 log BAU/mL for anti-spike IgG responses). No differences in antibody responses 4 weeks after the second dose were noted between the two vaccines, in either homologous or heterologous combinations.
Interpretation: Interim results of this ongoing longitudinal study show that among frail, older people, previous SARS-CoV-2 infection and the type of mRNA vaccine influenced antibody responses when used with a 16-week interval between doses. In these cohorts of frail, older individuals with a similar age and comorbidity distribution, we found that serological responses were similar and clinically equivalent between the discovery and confirmatory cohorts. Homologous and heterologous use of mRNA vaccines was not associated with significant differences in antibody responses 4 weeks following the second dose, supporting their interchangeability.
Funding: Public Health Agency of Canada, Vaccine Surveillance Reference Group; and the COVID-19 Immunity Task Force.
Translation: For the French translation of the abstract see Supplementary Materials section.
© 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license.
Conflict of interest statement
DCV is funded by the Fonds de la recherche en santé du Québec clinician-scientist scholar Junior 2 Program; has received clinical trial support from Cidara Therapeutics, CSL Behring, and Janssen Pharmaceuticals; has served on advisory boards for CSL Behring, Novartis Canada, and UCB Biosciences; has received speaker honoraria from CSL Behring and Merck Canada; and has a patent application pending (Electronic Filing System ID: 40101099) and a report of invention to McGill University (Track code: D2021-0043), both unrelated to this work. J-PG is funded by a Canada Research Chair award. Production of COVID-19 reagents was financially supported by National Research Council Canada's Pandemic Response Challenge Program. All other authors declare no competing interests.
Figures

Comment in
-
COVID-19 immunisation in older people.Lancet Healthy Longev. 2022 Mar;3(3):e126-e127. doi: 10.1016/S2666-7568(22)00036-8. Epub 2022 Feb 23. Lancet Healthy Longev. 2022. PMID: 35224523 Free PMC article. No abstract available.
References
-
- Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

