Tyrosine Kinases and Phosphatases: Enablers of the Porphyromonas gingivalis Lifestyle
- PMID: 35224543
- PMCID: PMC8863745
- DOI: 10.3389/froh.2022.835586
Tyrosine Kinases and Phosphatases: Enablers of the Porphyromonas gingivalis Lifestyle
Abstract
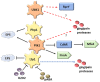
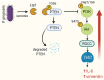
Tyrosine phosphorylation modifies the functionality of bacterial proteins and forms the basis of a versatile and tunable signal transduction system. The integrated action of tyrosine kinases and phosphatases controls bacterial processes important for metabolism and virulence. Porphyromonas gingivalis, a keystone pathogen in periodontal disease, possesses an extensive phosphotyrosine signaling network. The phosphorylation reaction is catalyzed by a bacterial tyrosine (BY) kinase, Ptk1, and a Ubiquitous bacterial Kinase UbK1. Dephosphorylation is mediated by a low-molecular-weight phosphatase, Ltp1 and a polymerase and histidinol phosphatase, Php1. Phosphotyrosine signaling controls exopolysaccharide production, gingipain activity, oxidative stress responses and synergistic community development with Streptococcus gordonii. Additionally, Ltp1 is secreted extracellularly and can be delivered inside gingival epithelial cells where it can override host cell signaling and readjust cellular physiology. The landscape of coordinated tyrosine kinase and phosphatase activity thus underlies the adaptive responses of P. gingivalis to both the polymicrobial environment of bacterial communities and the intracellular environment of gingival epithelial cells.
Keywords: Porphyromonas gingivalis; epithelial cells; polymicrobial; tyrosine kinase; tyrosine phosphatase.
Copyright © 2022 Lamont and Miller.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Porphyromonas gingivalis Tyrosine Phosphatase Php1 Promotes Community Development and Pathogenicity.mBio. 2019 Sep 24;10(5):e02004-19. doi: 10.1128/mBio.02004-19. mBio. 2019. PMID: 31551334 Free PMC article.
-
Characterization of a bacterial tyrosine kinase in Porphyromonas gingivalis involved in polymicrobial synergy.Microbiologyopen. 2014 Jun;3(3):383-94. doi: 10.1002/mbo3.177. Epub 2014 May 9. Microbiologyopen. 2014. PMID: 24811194 Free PMC article.
-
A bacterial tyrosine phosphatase modulates cell proliferation through targeting RGCC.PLoS Pathog. 2021 May 20;17(5):e1009598. doi: 10.1371/journal.ppat.1009598. eCollection 2021 May. PLoS Pathog. 2021. PMID: 34015051 Free PMC article.
-
Tyrosine phosphorylation and bacterial virulence.Int J Oral Sci. 2012 Mar;4(1):1-6. doi: 10.1038/ijos.2012.6. Epub 2012 Mar 2. Int J Oral Sci. 2012. PMID: 22388693 Free PMC article. Review.
-
Dual lifestyle of Porphyromonas gingivalis in biofilm and gingival cells.Microb Pathog. 2016 May;94:42-7. doi: 10.1016/j.micpath.2015.10.003. Epub 2015 Oct 9. Microb Pathog. 2016. PMID: 26456558 Review.
Cited by
-
Impact of Polymicrobial Infection on Fitness of Streptococcus gordonii In Vivo.mBio. 2023 Jun 27;14(3):e0065823. doi: 10.1128/mbio.00658-23. Epub 2023 Apr 12. mBio. 2023. PMID: 37042761 Free PMC article.
-
Modulation of myeloid-derived suppressor cell functions by oral inflammatory diseases and important oral pathogens.Front Immunol. 2024 Mar 1;15:1349067. doi: 10.3389/fimmu.2024.1349067. eCollection 2024. Front Immunol. 2024. PMID: 38495880 Free PMC article. Review.
-
Social networking at the microbiome-host interface.Infect Immun. 2023 Sep 14;91(9):e0012423. doi: 10.1128/iai.00124-23. Epub 2023 Aug 18. Infect Immun. 2023. PMID: 37594277 Free PMC article. Review.
-
The polymicrobial pathogenicity of Porphyromonas gingivalis.Front Oral Health. 2024 Apr 26;5:1404917. doi: 10.3389/froh.2024.1404917. eCollection 2024. Front Oral Health. 2024. PMID: 38736461 Free PMC article.
-
Connection between protein-tyrosine kinase inhibition and coping with oxidative stress in Bacillus subtilis.Proc Natl Acad Sci U S A. 2024 Jun 18;121(25):e2321890121. doi: 10.1073/pnas.2321890121. Epub 2024 Jun 10. Proc Natl Acad Sci U S A. 2024. PMID: 38857388 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

