Tyrosine Kinases and Phosphatases: Enablers of the Porphyromonas gingivalis Lifestyle
- PMID: 35224543
- PMCID: PMC8863745
- DOI: 10.3389/froh.2022.835586
Tyrosine Kinases and Phosphatases: Enablers of the Porphyromonas gingivalis Lifestyle
Abstract
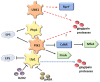
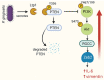
Tyrosine phosphorylation modifies the functionality of bacterial proteins and forms the basis of a versatile and tunable signal transduction system. The integrated action of tyrosine kinases and phosphatases controls bacterial processes important for metabolism and virulence. Porphyromonas gingivalis, a keystone pathogen in periodontal disease, possesses an extensive phosphotyrosine signaling network. The phosphorylation reaction is catalyzed by a bacterial tyrosine (BY) kinase, Ptk1, and a Ubiquitous bacterial Kinase UbK1. Dephosphorylation is mediated by a low-molecular-weight phosphatase, Ltp1 and a polymerase and histidinol phosphatase, Php1. Phosphotyrosine signaling controls exopolysaccharide production, gingipain activity, oxidative stress responses and synergistic community development with Streptococcus gordonii. Additionally, Ltp1 is secreted extracellularly and can be delivered inside gingival epithelial cells where it can override host cell signaling and readjust cellular physiology. The landscape of coordinated tyrosine kinase and phosphatase activity thus underlies the adaptive responses of P. gingivalis to both the polymicrobial environment of bacterial communities and the intracellular environment of gingival epithelial cells.
Keywords: Porphyromonas gingivalis; epithelial cells; polymicrobial; tyrosine kinase; tyrosine phosphatase.
Copyright © 2022 Lamont and Miller.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

