Population pharmacokinetics of piperacillin/tazobactam in critically ill Korean patients and the effects of extracorporeal membrane oxygenation
- PMID: 35224630
- PMCID: PMC9047688
- DOI: 10.1093/jac/dkac059
Population pharmacokinetics of piperacillin/tazobactam in critically ill Korean patients and the effects of extracorporeal membrane oxygenation
Abstract
Objectives: To explore extracorporeal membrane oxygenation (ECMO)-related alterations of the pharmacokinetics (PK) of piperacillin/tazobactam and determine an optimal dosage regimen for critically ill adult patients.
Methods: Population PK models for piperacillin/tazobactam were developed using a non-linear mixed effect modelling approach. The percentage of time within 24 h for which the free concentration exceeded the MIC at a steady-state (50%fT>MIC, 100%fT>MIC, and 100%fT>4×MIC) for various combinations of dosage regimens and renal function were explored using Monte-Carlo simulation.
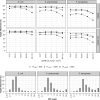
Results: A total of 226 plasma samples from 38 patients were used to develop a population PK model. Piperacillin/tazobactam PK was best described by two-compartment models, in which estimated glomerular filtration rate (eGFR), calculated using CKD-EPI equation based on cystatin C level, was a significant covariate for total clearance of each piperacillin and tazobactam. ECMO use decreased the central volume of distribution of both piperacillin and tazobactam in critically ill patients. Patients with Escherichia coli or Klebsiella pneumoniae infection, but not those with Pseudomonas aeruginosa infection, exhibited a PK/pharmacodynamic target attainment >90% when the target is 50%fT>MIC, as a result of applying the currently recommended dosage regimen. Prolonged or continuous infusion of 16 g/day was required when the treatment goal was 100%fT>MIC or 100%fT>4×MIC, and patients had an eGFR of 130-170 mL/min/1.73 m2.
Conclusions: ECMO use decreases piperacillin/tazobactam exposure. Prolonged or continuous infusion can achieve the treatment target in critically ill patients, particularly when MIC is above 8 mg/L or when patients have an eGFR of 130-170 mL/min/1.73 m2.
© The Author(s) 2022. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures




References
-
- De Waele JJ, Akova M, Antonelli Met al. . Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med 2018; 44: 189–96. - PubMed
-
- Smith BS, Yogaratnam D, Levasseur-Franklin KEet al. . Introduction to drug pharmacokinetics in the critically ill patient. Chest 2012; 141: 1327–36. - PubMed
-
- Varghese JM, Roberts JA, Lipman J. Antimicrobial pharmacokinetic and pharmacodynamic issues in the critically ill with severe sepsis and septic shock. Crit Care Clin 2011; 27: 19–34. - PubMed
-
- Shekar K, Fraser JF, Smith MTet al. . Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care 2012; 27: 741 e9–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

