A Canadian national guideline on the neoadjuvant treatment of invasive breast cancer, including patient assessment, systemic therapy, and local management principles
- PMID: 35224713
- PMCID: PMC8993711
- DOI: 10.1007/s10549-022-06522-6
A Canadian national guideline on the neoadjuvant treatment of invasive breast cancer, including patient assessment, systemic therapy, and local management principles
Abstract
Purpose: The neoadjuvant treatment of breast cancer (NABC) is a rapidly changing area that benefits from guidelines integrating evidence with expert consensus to help direct practice. This can optimize patient outcomes by ensuring the appropriate use of evolving neoadjuvant principles.
Methods: An expert panel formulated evidence-based practice recommendations spanning the entire neoadjuvant breast cancer treatment journey. These were sent for practice-based consensus across Canada using the modified Delphi methodology, through a secure online survey. Final recommendations were graded using the GRADE criteria for guidelines. The evidence was reviewed over the course of guideline development to ensure recommendations remained aligned with current relevant data.
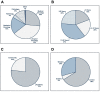
Results: Response rate to the online survey was almost 30%; representation was achieved from various medical specialties from both community and academic centres in various Canadian provinces. Two rounds of consensus were required to achieve 80% or higher consensus on 59 final statements. Five additional statements were added to reflect updated evidence but not sent for consensus.
Conclusions: Key highlights of this comprehensive Canadian guideline on NABC include the use of neoadjuvant therapy for early stage triple negative and HER2 positive breast cancer, with subsequent adjuvant treatments for patients with residual disease. The use of molecular signatures, other targeted adjuvant therapies, and optimal response-based local regional management remain actively evolving areas. Many statements had evolving or limited data but still achieved high consensus, demonstrating the utility of such a guideline in helping to unify practice while further evidence evolves in this important area of breast cancer management.
Keywords: Breast cancer; Consensus; Guideline; Neoadjuvant.
© 2022. The Author(s).
Conflict of interest statement
Primary Authors: SG—advisory board (Roche, Novartis, Lilly, Pfizer, Exact Science, and Agendia). External Reviewer: JFB—speaking honoraria (Roche, Novartis, Genomic Health, Pfizer, Allergan and Merck), consultant (Roche), Grants (Roche, Novartis, Pfzier, Abbvie), advisory board (Roche, Genomic Health, Nanostring Technologies, Pfizer, Lilly, Novartis and Merck). None of these COI were deemed impactful on the development or review of specific guideline recommendations.
Figures
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2021;71(3):209–249. - PubMed
-
- Canadian Cancer Society (2021) No title. https://www.cancer.ca/en/cancer-information/cancer-type/breast/prognosis.... Accessed 8 Mar 2021
-
- Prat A, Pineda E, Adamo B, Galván P, Fernández A, Gaba L, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. The Breast. 2015;24:S26–35. - PubMed
-
- Brackstone M, Robidoux A, Chia S, Mackey J, Dent R, Boileau J, et al. Canadian initiatives for locally advanced breast cancer research and treatment: inaugural meeting of the Canadian Consortium for LABC. Curr Oncol. 2011;18(3):139–144.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

