The innate immune stimulant Amplimune® is safe to administer to young feedlot cattle
- PMID: 35224736
- PMCID: PMC9306767
- DOI: 10.1111/avj.13156
The innate immune stimulant Amplimune® is safe to administer to young feedlot cattle
Abstract
Background: Infectious disease has a significant impact on livestock production. Availability of alternatives to antibiotics to prevent and treat disease is required to reduce reliance on antibiotics while not impacting animal welfare. Innate immune stimulants, such as mycobacterium cell wall fractions (MCWF), are used as alternatives to antibiotics for the treatment and prevention of infectious disease in a number of species including cattle, horses and dogs. This study aimed to evaluate the safety of Amplimune®, an MCWF-based immune stimulant, for weaner Angus cattle.
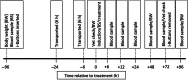
Methods: On day -1 and 0, sixty mixed-sex Angus weaner cattle were transported for 6 h before being inducted and housed in a large single pen, simulating feedlot induction conditions. The cattle were assigned to one of six treatment groups (n = 10 per group): 2 mL Amplimune intramuscularly (2IM); 2 mL Amplimune subcutaneously (2SC); 5 mL Amplimune intramuscularly (5IM); 5 mL Amplimune subcutaneously (5SC); 5 mL saline intramuscularly (SalIM) and 5 mL saline subcutaneously (SalSC) on day 0 following transportation. Body temperature, body weight, concentrations of circulating pro-inflammatory cytokines (TNFα, IL-1β, IL-6 and IL-12) and haematology parameters were measured at various times up to 96 h post-treatment.
Results: No adverse effects from Amplimune treatment were observed. Amplimune induced an increase in circulating cytokine TNFα concentrations, total white blood cell count and lymphocyte counts indicative of activation of the innate immune system without causing an excessive inflammatory response.
Conclusions: Results confirm that Amplimune can be safely administered to beef cattle at the dose rates and via the routes of administration investigated here.
Keywords: Amplimune; cattle; respiratory disease; trained immunity.
© 2022 The Authors. Australian Veterinary Journal published by John Wiley & Sons Australia, Ltd on behalf of Australian Veterinary Association.
Conflict of interest statement
The work was co‐funded by Meat and Livestock Australia (MLA) and the Commonwealth Scientific and Industrial Research Organisation (CSIRO). Author Annika Alexander was the recipient of the Ian McMaster Bequest scholarship and the QTAC Rural & Regional Enterprise scholarship. Authors McRae G, Alkemade S and Cervantes M were employees or are still employed by NovaVive (a company, which previously had or currently holds licensing rights for Amplimune™) at the time of study execution and manuscript preparation.
Figures
References
-
- Lane J, Jubb T, Shephard R, Webb‐Ware J, Fordyce G. Priority list of endemic diseases for the red meat industries . North Sydney, NSW; 2015. 20 March 2015. Contract No.: B.AHE.0010.
-
- Blecha F. Immune system response to stress. In: Moberg GP, Mench JA, editors. The biology of animal stress. CAB International, Oxford, 2000;111–122.
-
- Hay KE, Barnes TS, Morton JM et al. Risk factors for bovine respiratory disease in Australian feedlot cattle: use of a causal diagram‐informed approach to estimate effects of animal mixing and movements before feedlot entry.(clinical report). Prev Vet Med 2014;117(1):160–169. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources