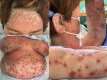
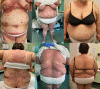
Generalized pustular psoriasis flare in a patient affected by plaque psoriasis after BNT162b2 mRNA COVID-19 vaccine, successfully treated with risankizumab
- PMID: 35224780
- PMCID: PMC9114914
- DOI: 10.1111/jdv.18032
Generalized pustular psoriasis flare in a patient affected by plaque psoriasis after BNT162b2 mRNA COVID-19 vaccine, successfully treated with risankizumab
Conflict of interest statement
A. Narcisi has served on advisory boards, received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi‐Genzyme, Amgen and Boehringer Ingelheim. A. Costanzo has been consultant and/or speaker for AbbVie, Almirall, Amgen, Janssen, Leo Pharma, Eli Lilly, Galderma, Boehringer, Novartis, Pfizer, Sandoz and UCB; R.G. Borroni has been consultant for Almirall and speaker for Abbvie.
Figures
Similar articles
-
De novo annular pustular psoriasis following mRNA COVID-19 vaccine.J Eur Acad Dermatol Venereol. 2022 Aug;36(8):e603-e605. doi: 10.1111/jdv.18114. Epub 2022 Apr 7. J Eur Acad Dermatol Venereol. 2022. PMID: 35349736 No abstract available.
-
COVID vaccine-induced pustular psoriasis in patients with previous plaque type psoriasis.J Eur Acad Dermatol Venereol. 2022 May;36(5):e330-e332. doi: 10.1111/jdv.17918. Epub 2022 Jan 28. J Eur Acad Dermatol Venereol. 2022. PMID: 35015916 No abstract available.
-
Exacerbations of generalized pustular psoriasis, palmoplantar psoriasis, and psoriasis vulgaris after mRNA COVID-19 vaccine: A report of three cases.Dermatol Ther. 2022 Apr;35(4):e15331. doi: 10.1111/dth.15331. Epub 2022 Feb 1. Dermatol Ther. 2022. PMID: 35066960 No abstract available.
-
Generalized pustular psoriasis (von Zumbusch).An Bras Dermatol. 2022 Jan-Feb;97(1):63-74. doi: 10.1016/j.abd.2021.05.011. Epub 2021 Nov 24. An Bras Dermatol. 2022. PMID: 34838431 Free PMC article. Review.
-
Generalized pustular psoriasis: the new era of treatment with IL-36 receptor inhibitors.J Dermatolog Treat. 2022 Nov;33(7):2911-2918. doi: 10.1080/09546634.2022.2089335. Epub 2022 Jun 21. J Dermatolog Treat. 2022. PMID: 35695278 Review.
Cited by
-
New-onset psoriasis after Comirnaty (BNT162b2, BioNTech/Pfizer) vaccine successfully treated with ixekizumab.Dermatol Ther. 2022 Aug;35(8):e15606. doi: 10.1111/dth.15606. Epub 2022 Jun 8. Dermatol Ther. 2022. PMID: 35635756 Free PMC article. No abstract available.
-
The Impact of COVID-19 Vaccination on Inflammatory Skin Disorders and Other Cutaneous Diseases: A Review of the Published Literature.Viruses. 2023 Jun 23;15(7):1423. doi: 10.3390/v15071423. Viruses. 2023. PMID: 37515110 Free PMC article. Review.
-
Cutaneous Reactions Following COVID-19 Vaccination: A Review of the Current Literature.Clin Cosmet Investig Dermatol. 2022 Nov 6;15:2369-2382. doi: 10.2147/CCID.S388245. eCollection 2022. Clin Cosmet Investig Dermatol. 2022. PMID: 36387962 Free PMC article. Review.
-
Interleukin-17 vs. Interleukin-23 Inhibitors in Pustular and Erythrodermic Psoriasis: A Retrospective, Multicentre Cohort Study.J Clin Med. 2023 Feb 19;12(4):1662. doi: 10.3390/jcm12041662. J Clin Med. 2023. PMID: 36836196 Free PMC article.
-
New Onset and Exacerbation of Psoriasis Following COVID-19 Vaccination: A Review of the Current Knowledge.Biomedicines. 2023 Aug 3;11(8):2191. doi: 10.3390/biomedicines11082191. Biomedicines. 2023. PMID: 37626687 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

