An Engineered Cytidine Deaminase for Biocatalytic Production of a Key Intermediate of the Covid-19 Antiviral Molnupiravir
- PMID: 35224970
- PMCID: PMC8915250
- DOI: 10.1021/jacs.1c11048
An Engineered Cytidine Deaminase for Biocatalytic Production of a Key Intermediate of the Covid-19 Antiviral Molnupiravir
Abstract
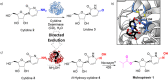
The Covid-19 pandemic highlights the urgent need for cost-effective processes to rapidly manufacture antiviral drugs at scale. Here we report a concise biocatalytic process for Molnupiravir, a nucleoside analogue recently approved as an orally available treatment for SARS-CoV-2. Key to the success of this process was the development of an efficient biocatalyst for the production of N-hydroxy-cytidine through evolutionary adaption of the hydrolytic enzyme cytidine deaminase. This engineered biocatalyst performs >85 000 turnovers in less than 3 h, operates at 180 g/L substrate loading, and benefits from in situ crystallization of the N-hydroxy-cytidine product (85% yield), which can be converted to Molnupiravir by a selective 5'-acylation using Novozym 435.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Painter G. R.; Bluemling G. R.; Natchus M. G.; Guthrie D.. N4-hydroxycytidine and derivatives and anti-viral uses related thereto. WO2019113462, 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

