Efficient SARS-CoV-2 Quantitative Reverse Transcriptase PCR Saliva Diagnostic Strategy utilizing Open-Source Pipetting Robots
- PMID: 35225290
- PMCID: PMC9199378
- DOI: 10.3791/63395
Efficient SARS-CoV-2 Quantitative Reverse Transcriptase PCR Saliva Diagnostic Strategy utilizing Open-Source Pipetting Robots
Abstract
The emergence of the recent SARS-CoV-2 global health crisis introduced key challenges for epidemiological research and clinical testing. Characterized by a high rate of transmission and low mortality, the COVID-19 pandemic necessitated accurate and efficient diagnostic testing, particularly in closed populations such as residential universities. Initial availability of nucleic acid testing, like nasopharyngeal swabs, was limited due to supply chain pressure which also delayed reporting of test results. Saliva-based reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) testing has shown to be comparable in sensitivity and specificity to other testing methods, and saliva collection is less physically invasive to participants. Consequently, we developed a multiplex RT-qPCR diagnostic assay for population surveillance of Clemson University and the surrounding community. The assay utilized open-source liquid handling robots and thermocyclers instead of complex clinical automation systems to optimize workflow and system flexibility. Automation of saliva-based RT-qPCR enables rapid and accurate detection of a wide range of viral RNA concentrations for both large- and small-scale testing demands. The average turnaround for the automated system was < 9 h for 95% of samples and < 24 h for 99% of samples. The cost for a single test was $2.80 when all reagents were purchased in bulk quantities.
Figures




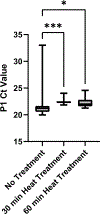
References
-
- World Health Organization. Surveillance strategies for COVID-19 human infection: interim guidance, 10 May 2020. World Health Organization. https://apps.who.int/iris/handle/10665/332051 (2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
