Oxidation of BINOLs by Hypervalent Iodine Reagents: Facile Synthesis of Xanthenes and Lactones
- PMID: 35225370
- PMCID: PMC9311707
- DOI: 10.1002/chem.202200181
Oxidation of BINOLs by Hypervalent Iodine Reagents: Facile Synthesis of Xanthenes and Lactones
Abstract
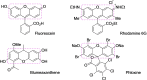
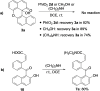
Xanthene derivatives have broad applications in medicines, fluorescent probes, dyes, food additives, etc. Therefore, much attention was focused on developing the synthetic methods to prepare these compounds. Binaphthyl-based xanthene derivatives were prepared through the oxidation of BINOLs promoted by the hypervalent iodine reagent iodosylbenzene (PhIO). Nine-membered lactones were obtained through a similar oxidative reaction when iodoxybenzene (PhIO2 ) was used. Additionally, one-pot reactions of BINOLs, PhIO and nucleophiles such as alcohols and amines were also investigated to provide alkoxylated products and amides in good to excellent yields.
Keywords: BINOL; hypervalent iodine reagents; lactones; oxidation; xanthenes.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- None
-
- Guo S.-H., Leng T.-H., Wang K., Wang C.-Y., Shen Y.-J., Zhu W.-H., Talanta 2018, 185, 359–364; - PubMed
-
- Liu J., Chen X., Zhang Y., Gao G., Zhang X., Hou S., J. Lumin. 2018, 204, 480–484;
-
- Zhang N., Dong B., Kong X., Wang C., Song W., Lin W., J. Fluoresc. 2018, 28, 681–687; - PubMed
-
- Ebaston T. M., Rozovsky A., Zaporozhets A., Bazylevich A., Tuchinsky H., Marks V., Gellerman G., Patsenker L. D., ChemMedChem 2019, 14, 1727–1734; - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

