Surface Structure of Alkyl/Fluoroalkylimidazolium Ionic-Liquid Mixtures
- PMID: 35225614
- PMCID: PMC9007465
- DOI: 10.1021/acs.jpcb.1c10460
Surface Structure of Alkyl/Fluoroalkylimidazolium Ionic-Liquid Mixtures
Abstract
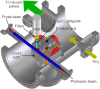
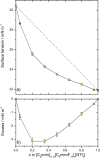
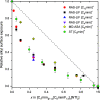
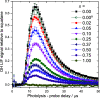
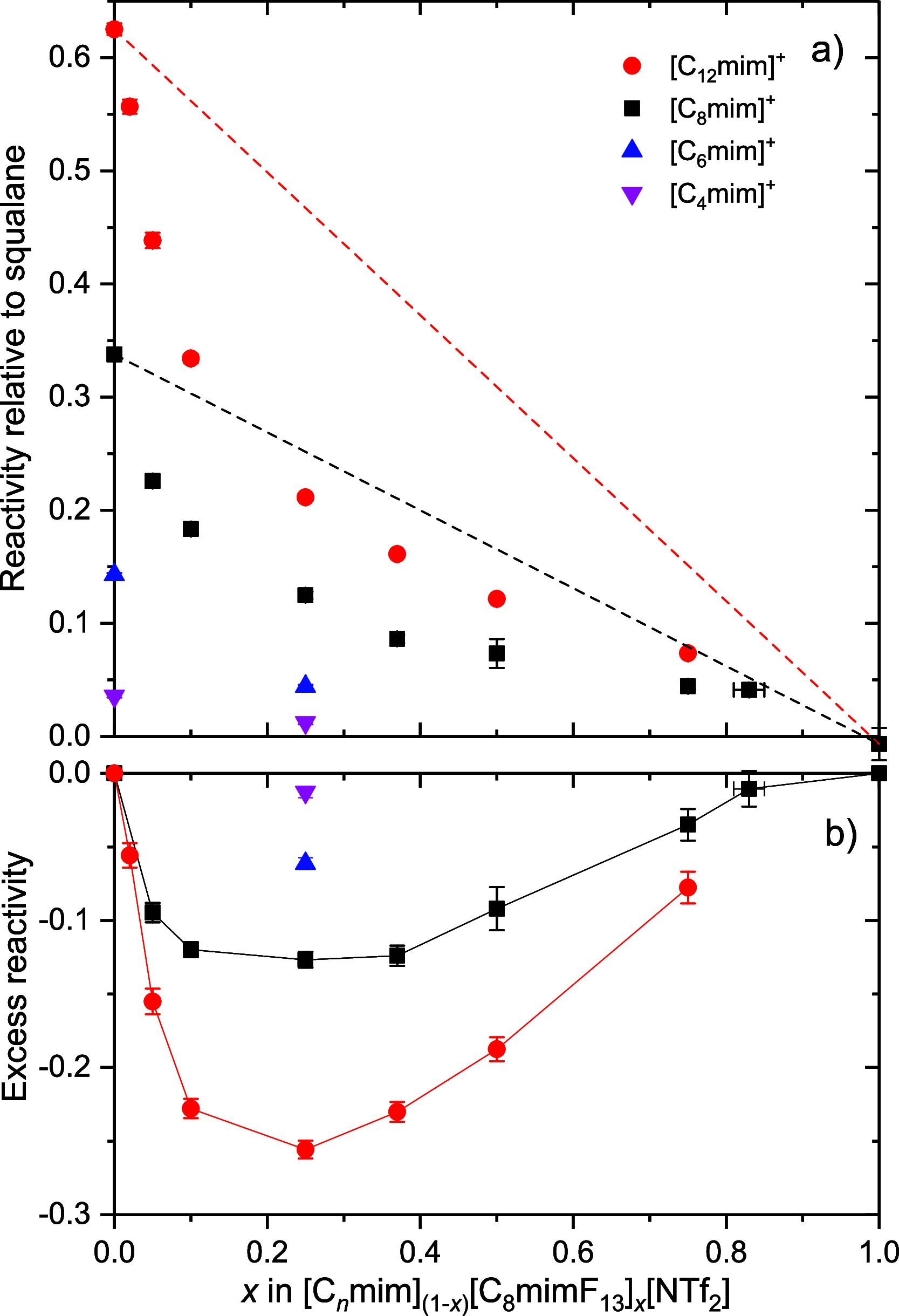
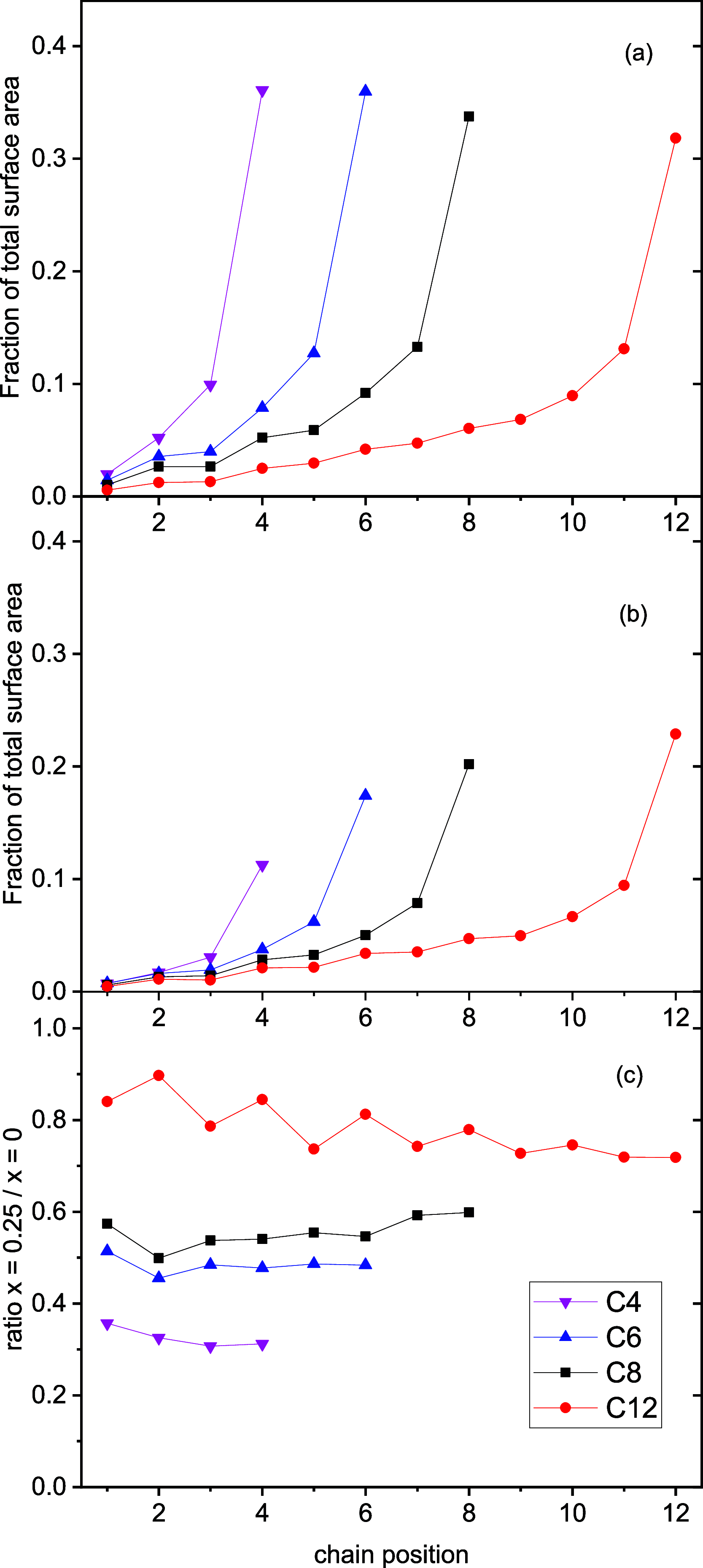
The gas-liquid interface of ionic liquids (ILs) is critically important in many applications, for example, in supported IL phase (SILP) catalysis. Methods to investigate the interfacial structure in these systems will allow their performance to be improved in a rational way. In this study, reactive-atom scattering (RAS), surface tension measurements, and molecular dynamics (MD) simulations were used to study the vacuum interface of mixtures of partially fluorinated and normal alkyl ILs. The underlying aim was to understand whether fluorinated IL ions could be used as additives to modify the surface structure of one of the most widely used families of alkyl ILs. The series of ILs 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([Cnmim][Tf2N]) with n = 4-12 were mixed with a fixed-length, semiperfluorinated analogue (1H,1H,2H,2H-perfluorooctyl)-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([C8mimF13][Tf2N]), forming [Cnmim](1-x)[C8mimF13]x[Tf2N] mixtures, where x is the bulk mole fraction of the fluorinated component. The RAS-LIF method combined O-atom projectiles with laser-induced fluorescence (LIF) detection of the product OH as a measure of surface exposure of the alkyl chains. For [C8mim](1-x)[C8mimF13]x[Tf2N] mixtures, RAS-LIF OH yields are below those expected from stoichiometry. There are quantitatively consistent negative deviations from linearity of the surface tension. Both results imply that the lower-surface-tension fluoroalkyl material dominates the surface. A similar deficit is found for alkyl chain lengths n = 4, 6, 8, and 12 and for all (nonzero) x investigated by RAS-LIF. Accessible-surface-area (ASA) analyses of the MD simulations for [Cnmim](1-x)[C8mimF13]x[Tf2N] mixtures qualitatively reproduce the same primary effect of fluoro-chain predominance of the surface over most of the range of n. However, there are significant quantitative discrepancies between MD ASA predictions and experiment relating to the strength of any n-dependence of the relative alkyl coverage at fixed x, and on the x-dependence at fixed n. These discrepancies are discussed in the context of detailed examinations of the surface structures predicted in the MD simulations. Potential explanations, beyond experimental artifacts, include inadequacies in the classical force fields used in the MD simulations or the inability of simple ASA algorithms to capture dynamical factors that influence RAS-LIF yields.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Zhao D.; Wu M.; Kou Y.; Min E. Ionic Liquids: Applications in Catalysis. Catal. Today 2002, 74, 157–189. 10.1016/s0920-5861(01)00541-7. - DOI
-
- Sheldon R. A.; Lau R. M.; Sorgedrager M. J.; van Rantwijk F.; Seddon K. R. Biocatalysis in Ionic Liquids. Green Chem. 2002, 4, 147–151. 10.1039/b110008b. - DOI
-
- Welton T. Ionic Liquids in Catalysis. Coord. Chem. Rev. 2004, 248, 2459–2477. 10.1016/j.ccr.2004.04.015. - DOI
-
- Giernoth R.Homogeneous Catalysis in Ionic Liquids. In In Situ NMR Methods in Catalysis; Springer; Bargon J., Kuhn L. T., Eds., 2007; Vol. 276, pp 1–23.
LinkOut - more resources
Full Text Sources
Miscellaneous

