Differentiation of Cells Isolated from Human Femoral Heads into Functional Osteoclasts
- PMID: 35225960
- PMCID: PMC8883933
- DOI: 10.3390/jdb10010006
Differentiation of Cells Isolated from Human Femoral Heads into Functional Osteoclasts
Abstract
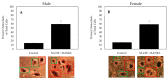
Proper formation of the skeleton during development is crucial for the mobility of humans and the maintenance of essential organs. The production of bone is regulated by osteoblasts and osteoclasts. An imbalance of these cells can lead to a decrease in bone mineral density, which leads to fractures. While many studies are emerging to understand the role of osteoblasts, less studies are present about the role of osteoclasts. This present study utilized bone marrow cells isolated directly from the bone marrow of femoral heads obtained from osteoarthritic (OA) patients after undergoing hip replacement surgery. Here, we used tartrate resistant acid phosphatase (TRAP) staining, Cathepsin K, and nuclei to identity osteoclasts and their functionality after stimulation with macrophage-colony stimulation factor (M-CSF) and receptor activator of nuclear factor kappa-β ligand (RANKL). Our data demonstrated that isolated cells can be differentiated into functional osteoclasts, as indicated by the 92% and 83% of cells that stained positive for TRAP and Cathepsin K, respectively. Furthermore, isolated cells remain viable and terminally differentiate into osteoclasts when stimulated with RANKL. These data demonstrate that cells isolated from human femoral heads can be differentiated into osteoclasts to study bone disorders during development and adulthood.
Keywords: Cathepsin K; M-CSF; RANKL; RAW 264.7 cells; TRAP; bone; osteoarthritis; osteoclasts; osteoporosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

