Biomimetic PLGA/Strontium-Zinc Nano Hydroxyapatite Composite Scaffolds for Bone Regeneration
- PMID: 35225976
- PMCID: PMC8883951
- DOI: 10.3390/jfb13010013
Biomimetic PLGA/Strontium-Zinc Nano Hydroxyapatite Composite Scaffolds for Bone Regeneration
Abstract
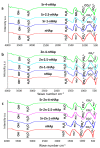
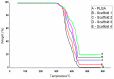
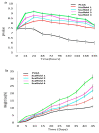
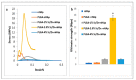
Synthetic bone graft substitutes have attracted increasing attention in tissue engineering. This study aimed to fabricate a novel, bioactive, porous scaffold that can be used as a bone substitute. Strontium and zinc doped nano-hydroxyapatite (Sr/Zn n-HAp) were synthesized by a water-based sol-gel technique. Sr/Zn n-HAp and poly (lactide-co-glycolide) (PLGA) were used to fabricate composite scaffolds by supercritical carbon dioxide technique. FTIR, XRD, TEM, SEM, and TGA were used to characterize Sr/Zn n-HAp and the composite scaffolds. The synthesized scaffolds were adequately porous with an average pore size range between 189 to 406 µm. The scaffolds demonstrated bioactive behavior by forming crystals when immersed in the simulated body fluid. The scaffolds after immersing in Tris/HCl buffer increased the pH value of the medium, establishing their favorable biodegradable behavior. ICP-MS study for the scaffolds detected the presence of Sr, Ca, and Zn ions in the SBF within the first week, which would augment osseointegration if implanted in the body. nHAp and their composites (PLGA-nHAp) showed ultimate compressive strength ranging between 0.4-19.8 MPa. A 2.5% Sr/Zn substituted nHAp-PLGA composite showed a compressive behavior resembling that of cancellous bone indicating it as a good candidate for cancellous bone substitute.
Keywords: PLGA; bone scaffolds; nano-hydroxyapatite; strontium; supercritical CO2; zinc.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures











References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

