Neurologic Prognostication After Cardiac Arrest Using Brain Biomarkers: A Systematic Review and Meta-analysis
- PMID: 35226054
- PMCID: PMC8886448
- DOI: 10.1001/jamaneurol.2021.5598
Neurologic Prognostication After Cardiac Arrest Using Brain Biomarkers: A Systematic Review and Meta-analysis
Abstract
Importance: Brain injury biomarkers released into circulation from the injured neurovascular unit are important prognostic tools in patients with cardiac arrest who develop hypoxic ischemic brain injury (HIBI) after return of spontaneous circulation (ROSC).
Objective: To assess the neuroprognostic utility of bloodborne brain injury biomarkers in patients with cardiac arrest with HIBI.
Data sources: Studies in electronic databases from inception to September 15, 2021. These databases included MEDLINE, Embase, Evidence-Based Medicine Reviews, CINAHL, Cochrane Database of Systematic Reviews, and the World Health Organization Global Health Library.
Study selection: Articles included in this systmatic review and meta-analysis were independently assessed by 2 reviewers. We included studies that investigated neuron-specific enolase, S100 calcium-binding protein β, glial fibrillary acidic protein, neurofilament light, tau, or ubiquitin carboxyl hydrolase L1 in patients with cardiac arrest aged 18 years and older for neurologic prognostication. We excluded studies that did not (1) dichotomize neurologic outcome as favorable vs unfavorable, (2) specify the timing of blood sampling or outcome determination, or (3) report diagnostic test accuracy or biomarker concentration.
Data extraction and synthesis: Data on the study design, inclusion and exclusion criteria, brain biomarkers levels, diagnostic test accuracy, and neurologic outcome were recorded. This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.
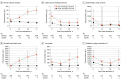
Main outcomes and measures: Summary receiver operating characteristic curve analysis was used to calculate the area under the curve, sensitivity, specificity, and optimal thresholds for each biomarker. Risk of bias and concerns of applicability were assessed with the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool.
Results: We identified 2953 studies, of which 86 studies with 10 567 patients (7777 men [73.6] and 2790 women [26.4]; pooled mean [SD] age, 62.8 [10.2] years) were included. Biomarker analysis at 48 hours after ROSC demonstrated that neurofilament light had the highest predictive value for unfavorable neurologic outcome, with an area under the curve of 0.92 (95% CI, 0.84-0.97). Subgroup analyses of patients treated with targeted temperature management and those who specifically had an out-of-hospital cardiac arrest showed similar results (targeted temperature management, 0.92 [95% CI, 0.86-0.95] and out-of-hospital cardiac arrest, 0.93 [95% CI, 0.86-0.97]).
Conclusions and relevance: Neurofilament light, which reflects white matter damage and axonal injury, yielded the highest accuracy in predicting neurologic outcome in patients with HIBI at 48 hours after ROSC.
Trial registration: PROSPERO Identifier: CRD42020157366.
Conflict of interest statement
Figures