Quality of Life Following Receipt of Adjuvant Chemotherapy With and Without Bevacizumab in Patients With Lymph Node-Positive and High-Risk Lymph Node-Negative Breast Cancer
- PMID: 35226083
- PMCID: PMC8886546
- DOI: 10.1001/jamanetworkopen.2022.0254
Quality of Life Following Receipt of Adjuvant Chemotherapy With and Without Bevacizumab in Patients With Lymph Node-Positive and High-Risk Lymph Node-Negative Breast Cancer
Abstract
Importance: Breast cancer treatment can impact not only short-term health but may also affect longer-term quality of life (QOL).
Objective: To describe and evaluate factors associated with diminished QOL following completion of active treatment.
Design, setting, and participants: This was a secondary analysis of a randomized clinical trial included patients with lymph node-positive or high-risk lymph node-negative breast cancer who had undergone definitive surgery and were enrolled in ECOG-ACRIN E5103, a multisite phase 3 trial. A survey was administered 18 months after enrollment to patients enrolled between January and June 2010. Final analysis of the data took place from March to December 2021.
Interventions: Patients received adjuvant doxorubicin, cyclophosphamide, and paclitaxel with either bevacizumab or placebo.
Main outcomes and measures: QOL and health status assessed with the EuroQol 5-Dimension 3-Levels (EQ-5D-3L), EQ-visual analog scale (EQ-VAS), and the Functional Assessment of Cancer Therapy-Breast Cancer, with arm subscale (FACT-B+4). Groups were compared by Fisher exact test, Wilcoxon rank sum, or Kruskal-Wallis test. Multivariable linear regression was used to assess factors independently associated with FACT-B scores.
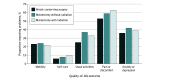
Results: Data at 18 months were available from 455 of 519 patients (87.7%) enrolled in the trial. Median (range) age at enrollment was 52 (25-76) years. No differences in QOL (median [range] FACT-B scores: group A, 123 [67-146]; group B, 114 [54-148]; group C, 117 [42-148]; P = .23) or health status (median [range] EQ-5D-3L index scores: group A, 0.83 [0.28-1.00]; group B, 0.83 [0.20-1.00]; group C, 0.83 [0.17-1.00], P = .80; median EQ-VAS: group A, 85 [20-100]; group B, 85 [0-100]; group C, 85 [0-100]; P = .79) were observed across treatment groups; results for subsequent analyses were therefore reported irrespective of primary treatment. Overall, half of patients (258 of 444 [58%]) reported at least some pain or discomfort; 170 (38%) reported symptoms of anxiety or depression. In multivariable analyses, mastectomy with radiation (vs breast conserving surgery) and Asian, Black, or American Indian or Alaska Native race (vs White race) were associated with lower QOL (mastectomy with radiation: coefficient: -5.5; 95% CI, -10.1 to -0.9; Asian, Black, or American Indian or Alaska Native race: coefficient: -7.3; 95% CI, -13.2, -1.4).
Conclusions and relevance: In this study, the addition of bevacizumab to chemotherapy was not negatively associated with QOL at 18 months. A substantial proportion of participants reported problems related to pain or discomfort and anxiety or depression, demonstrating persistent consequences for physical and psychosocial well-being in this heavily treated population. Many problems reported are amenable to intervention, underscoring the need for timely referral to supportive resources, especially for women of color and those who have more extensive local therapy.
Trial registration: ClinicalTrials.gov Identifier: NCT00433511.
Conflict of interest statement
Figures
References
-
- Miller KD, O’Neill A, Gradishar W, et al. . Double-blind phase III trial of adjuvant chemotherapy with and without bevacizumab in patients with lymph node-positive and high-risk lymph node-negative breast cancer (E5103). J Clin Oncol. 2018;36(25):2621-2629. doi:10.1200/JCO.2018.79.2028 - DOI - PMC - PubMed

