Association of the Timing of Postpartum Intrauterine Device Insertion and Breastfeeding With Risks of Intrauterine Device Expulsion
- PMID: 35226086
- PMCID: PMC8886522
- DOI: 10.1001/jamanetworkopen.2021.48474
Association of the Timing of Postpartum Intrauterine Device Insertion and Breastfeeding With Risks of Intrauterine Device Expulsion
Abstract
Importance: Intrauterine device (IUD) expulsion increases the risk of unintended pregnancy; how timing of postpartum IUD insertion and breastfeeding are associated with risk of expulsion is relevant to the benefit-risk profile.
Objective: To evaluate the association of postpartum timing of IUD insertion and breastfeeding status with incidence and risk of IUD expulsion.
Design, setting, and participants: The Association of Perforation and Expulsion of Intrauterine Devices (APEX-IUD) cohort study included women aged 50 years or younger with an IUD insertion between 2001 and 2018. The breastfeeding analysis focused on a subcohort of women at 52 or fewer weeks post partum with known breastfeeding status. The study was conducted using data from electronic health records (EHRs) at 4 research sites with access to EHR: 3 Kaiser Permanente sites (Northern California, Southern California, Washington) and the Regenstrief Institute (Indiana). Data analysis was conducted from June to November 2019.
Exposures: Timing of IUD insertion post partum was categorized into discrete time periods: 0 to 3 days, 4 days to 6 or fewer weeks, more than 6 weeks to 14 or fewer weeks, more than 14 weeks to 52 or fewer weeks, and non-post partum (>52 weeks or no evidence of delivery). Breastfeeding status at the time of insertion was determined from clinical records, diagnostic codes, or questionnaires from well-baby visits.
Main outcomes and measures: Incidence rates and adjusted hazard ratios (aHRs) were estimated using propensity scores to adjust for confounding.
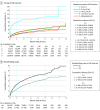
Results: The full cohort included 326 658 women (mean [SD] age, 32.0 [8.3] years; 38 911 [11.9%] Asian or Pacific Islander; 696 [0.2%] Hispanic Black; 56 180 [17.2%] Hispanic other; 42 501 [13.0%] Hispanic White; 28 323 [8.7%] non-Hispanic Black; 137 102 [42.0%] non-Hispanic White), and the subcohort included 94 817 women. Most IUDs were levonorgestrel-releasing (259 234 [79.4%]). There were 8943 expulsions. The 5-year cumulative incidence of IUD expulsion was highest for insertions 0 to 3 days post partum (10.73%; 95% CI, 9.12%-12.61%) and lowest for insertions more than 6 weeks to 14 or fewer weeks post partum (3.18%; 95% CI, 2.95%-3.42%). Adjusted HRs using women with non-post partum IUD insertion as the referent were 5.34 (95% CI, 4.47-6.39) for those with postpartum insertion at 0 to 3 days; 1.22 (95% CI, 1.05-1.41) for those with postpartum insertion at 4 days to 6 or fewer weeks; 1.06 (95% CI, 0.95-1.18) for those with postpartum insertion at more than 6 to 14 or fewer weeks; and 1.43 (95% CI, 1.29-1.60) for those with postpartum insertion at more than 14 to 52 or fewer weeks. In the subcohort, 5-year cumulative incidence was 3.49% (95% CI, 3.25%-3.73%) for breastfeeding women and 4.57% (95% CI, 4.22%-4.95%) for nonbreastfeeding women; the adjusted HR for breastfeeding vs not breastfeeding was 0.71 (95% CI, 0.64-0.78).
Conclusions and relevance: In this study of real-world data, IUD expulsion was rare but more common with immediate postpartum insertion. Breastfeeding was associated with lower expulsion risk.
Conflict of interest statement
Figures



References
-
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223(2):177-188. doi: 10.1016/j.ajog.2020.02.045 - DOI - PMC - PubMed
-
- Cunningham FG, Leveno KJ, Bloom SL, et al. Williams Obstetrics. 25th ed. McGraw Hill; 2018.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

