Distinct Roles of Rodent Thalamus and Corpus Callosum in Seizure Generalization
- PMID: 35226367
- PMCID: PMC9315027
- DOI: 10.1002/ana.26338
Distinct Roles of Rodent Thalamus and Corpus Callosum in Seizure Generalization
Abstract
Objective: Bilateral synchronous cortical activity occurs during sleep, attention, and seizures. Canonical models place the thalamus at the center of bilateral cortical synchronization because it generates bilateral sleep spindle oscillations and primarily generalized absence seizures. However, classical studies suggest that the corpus callosum mediates bilateral cortical synchronization.
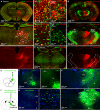
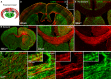
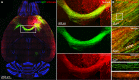
Methods: We mapped the spread of right frontal lobe-onset, focal to bilateral seizures in mice and modified it using chemo and optogenetic suppression of motor thalamic nucleus and corpus callosotomy.
Results: Seizures from the right cortex spread faster to the left cortex than to the left thalamus. The 2 thalami have minimal monosynaptic commissural connections compared to the massive commissure corpus callosum. Chemogenetic and closed-loop optogenetic inhibition of the right ventrolateral thalamic nucleus did not alter inter-hemispheric seizure spread. However, anterior callosotomy delayed bilateral seizure oscillations.
Interpretation: Thalamocortical oscillations amplify focal onset motor seizures, and corpus callosum spreads them bilaterally. ANN NEUROL 2022;91:682-696.
© 2022 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
The authors report no conflicts of interests.
Figures




Comment in
-
Focal Seizures Cruise the Callosal Highway.Epilepsy Curr. 2022 Sep 22;22(6):381-383. doi: 10.1177/15357597221125220. eCollection 2022 Nov-Dec. Epilepsy Curr. 2022. PMID: 36426194 Free PMC article. No abstract available.
References
-
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science 1993;262:679–685. - PubMed
-
- Blumenfeld H. The thalamus and seizures. Arch Neurol 2002;59:135–137. - PubMed
-
- Huguenard JR, Prince DA. Basic mechanisms of epileptic discharges in the thalamus. In: Steriade M, Jones EG, McCormick DA, eds. Thalamus. Amsterdam, Netherlands: Elsevier, 1997:295‐330.
-
- Penfield W, Jasper HH. Epilepsy and the Functional Anatomy of the Human Brain. Boston: Little, Brown, 1954.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

