Remote blood collection from older adults in the Brain Health Registry for plasma biomarker and genetic analysis
- PMID: 35226409
- PMCID: PMC9998146
- DOI: 10.1002/alz.12617
Remote blood collection from older adults in the Brain Health Registry for plasma biomarker and genetic analysis
Abstract
Introduction: Use of online registries to efficiently identify older adults with cognitive decline and Alzheimer's disease (AD) is an approach with growing evidence for feasibility and validity. Linked biomarker and registry data can facilitate AD clinical research.
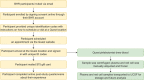
Methods: We collected blood for plasma biomarker and genetic analysis from older adult Brain Health Registry (BHR) participants, evaluated feasibility, and estimated associations between demographic variables and study participation.
Results: Of 7150 participants invited to the study, 864 (12%) enrolled and 629 (73%) completed remote blood draws. Participants reported high study acceptability. Those from underrepresented ethnocultural and educational groups were less likely to participate.
Discussion: This study demonstrates the challenges of remote blood collection from a large representative sample of older adults. Remote blood collection from > 600 participants within a short timeframe demonstrates the feasibility of our approach, which can be expanded for efficient collection of plasma AD biomarker and genetic data.
Keywords: Alzheimer's disease; acceptability; aging research; brain health registry; education; engagement; ethnicity; feasibility; genetics; internet; plasma biomarkers; race; research registry.
© 2022 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Miriam Ashford, Joseph Eichenbaum, Aniekan Akenam, Derek Flenniken, and Alexander Happ have no conflicts of interest to declare. Juliet Focker has received support from the California Department of Public Health. Taylor Howell has received support for attendance to AAIC from UCSF and support for attendance to CTAD from NCIRE. Diana Truran serves as an NCIRE Audit Committee advisor (unpaid). Scott Mackin has received NIH grant funding for institutional support. Kaj Blennow has received support from Abcam, Axon, Biogen, JOMDD/Shimadzu, Lilly, MagQu, Prothena, GEECD/Roche Diagnostics, Siemens Healthineers, IFCC/SNIBE, Julius Clinical, and Novartis. He is the co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. Eran Halperin has received support from the NIH, NSF, and consulted for KHealth and Ultima Genomics. Giovanni Coppola is a full‐time employee of Regeneron Pharmaceuticals. Michael Weiner receives support for his work from the following funding sources: NIH, Department of Defense, California Department of Public Health, University of Michigan, Siemens, Biogen, Larry L. Hillblom Foundation, Alzheimer's Association, and the State of California. He also receives support from Johnson & Johnson, Kevin and Connie Shanahan, VUmc, GE, Australian Catholic University, the Stroke Foundation, and the Veterans Administration. He has served on Advisory Boards for Cerecin/Accera, Roche, Alzheon, Inc., Merck Sharp & Dohme Corp., Nestle/Nestec, PCORI/PPRN, Dolby Family Ventures, National Institute on Aging (NIA), Brain Health Registry, and ADNI. He serves on the editorial boards for
Figures
References
-
- Zhong K, Cummings J. Healthybrains.org: from registry to randomization. J Prev Alzheimers Dis. 2016;3(3):123‐126. - PubMed
-
- Grill JD, Hoang D, Gillen DL, et al. Constructing a local potential participant registry to improve Alzheimer's disease clinical research recruitment. J Alzheimers Dis. 2018;63(3):1055‐1063. - PubMed