Major Update 2: Remdesivir for Adults With COVID-19: A Living Systematic Review and Meta-analysis for the American College of Physicians Practice Points
- PMID: 35226522
- PMCID: PMC8924790
- DOI: 10.7326/M21-4784
Major Update 2: Remdesivir for Adults With COVID-19: A Living Systematic Review and Meta-analysis for the American College of Physicians Practice Points
Abstract
Background: Remdesivir is approved for the treatment of adults hospitalized with COVID-19.
Purpose: To update a living review of remdesivir for adults with COVID-19.
Data sources: Several electronic U.S. Food and Drug Administration, company, and journal websites from 1 January 2020 through 19 October 2021.
Study selection: English-language, randomized controlled trials (RCTs) of remdesivir for COVID-19.
Data extraction: One reviewer abstracted, and a second reviewer verified data. The Cochrane Risk of Bias Tool and GRADE (Grading of Recommendations Assessment, Development and Evaluation) method were used.
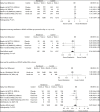
Data synthesis: Since the last update (search date 9 August 2021), 1 new RCT and 1 new subtrial comparing a 10-day course of remdesivir with control (placebo or standard care) were identified. This review summarizes and updates the evidence on the cumulative 5 RCTs and 2 subtrials for this comparison. Our updated results confirm a 10-day course of remdesivir, compared with control, probably results in little to no mortality reduction (5 RCTs). Updated results also confirm that remdesivir probably results in a moderate increase in the proportion of patients recovered by day 29 (4 RCTs) and may reduce time to clinical improvement (2 RCTs) and hospital length of stay (4 RCTs). New RCTs, by increasing the strength of evidence, lead to an updated conclusion that remdesivir probably results in a small reduction in the proportion of patients receiving ventilation or extracorporeal membrane oxygenation at specific follow-up times (4 RCTs). New RCTs also alter the conclusions for harms-remdesivir, compared with control, may lead to a small reduction in serious adverse events but may lead to a small increase in any adverse event.
Limitation: The RCTs differed in definitions of COVID-19 severity and outcomes reported.
Conclusion: In hospitalized adults with COVID-19, the findings confirm that remdesivir probably results in little to no difference in mortality and increases the proportion of patients recovered. Remdesivir may reduce time to clinical improvement and may lead to small reductions in serious adverse events but may result in a small increase in any adverse event.
Primary funding source: U.S. Department of Veterans Affairs.
Conflict of interest statement
Figures
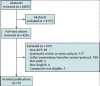

Update of
-
Major Update: Remdesivir for Adults With COVID-19 : A Living Systematic Review and Meta-analysis for the American College of Physicians Practice Points.Ann Intern Med. 2021 May;174(5):663-672. doi: 10.7326/M20-8148. Epub 2021 Feb 9. Ann Intern Med. 2021. Update in: Ann Intern Med. 2021 Dec;174(12):W114-W115. doi: 10.7326/L21-0600. Update in: Ann Intern Med. 2022 May;175(5):701-709. doi: 10.7326/M21-4784. PMID: 33560863 Free PMC article. Updated.
Comment in
-
Major Update 2: Remdesivir for Adults With COVID-19.Ann Intern Med. 2022 Aug;175(8):W81. doi: 10.7326/L22-0211. Ann Intern Med. 2022. PMID: 35969872 No abstract available.
-
Major Update 2: Remdesivir for Adults With COVID-19.Ann Intern Med. 2022 Aug;175(8):W81-W82. doi: 10.7326/L22-0212. Ann Intern Med. 2022. PMID: 35969873 No abstract available.
References
-
- U.S. Food and Drug Administration. FDA's approval of Veklury (remdesivir) for the treatment of COVID-19—the science of safety and effectiveness. Accessed at www.fda.gov/drugs/news-events-human-drugs/fdas-approval-veklury-remdesiv... on 14 November 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
