BET-bromodomain and EZH2 inhibitor-treated chronic GVHD mice have blunted germinal centers with distinct transcriptomes
- PMID: 35226736
- PMCID: PMC9101246
- DOI: 10.1182/blood.2021014557
BET-bromodomain and EZH2 inhibitor-treated chronic GVHD mice have blunted germinal centers with distinct transcriptomes
Erratum in
-
Zaiken MC, Flynn R, Paz KG, et al. BET-bromodomain and EZH2 inhibitor-treated chronic GVHD mice have blunted germinal centers with distinct transcriptomes. Blood. 2022;139(19):2983-2997.Blood. 2023 Oct 19;142(16):1405. doi: 10.1182/blood.2023022398. Blood. 2023. PMID: 37856091 Free PMC article. No abstract available.
Abstract
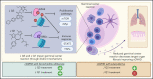
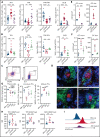
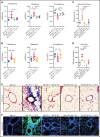
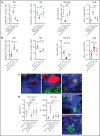
Despite advances in the field, chronic graft-versus-host-disease (cGVHD) remains a leading cause of morbidity and mortality following allogenic hematopoietic stem cell transplant. Because treatment options remain limited, we tested efficacy of anticancer, chromatin-modifying enzyme inhibitors in a clinically relevant murine model of cGVHD with bronchiolitis obliterans (BO). We observed that the novel enhancer of zeste homolog 2 (EZH2) inhibitor JQ5 and the BET-bromodomain inhibitor JQ1 each improved pulmonary function; impaired the germinal center (GC) reaction, a prerequisite in cGVHD/BO pathogenesis; and JQ5 reduced EZH2-mediated H3K27me3 in donor T cells. Using conditional EZH2 knockout donor cells, we demonstrated that EZH2 is obligatory for the initiation of cGVHD/BO. In a sclerodermatous cGVHD model, JQ5 reduced the severity of cutaneous lesions. To determine how the 2 drugs could lead to the same physiological improvements while targeting unique epigenetic processes, we analyzed the transcriptomes of splenic GCB cells (GCBs) from transplanted mice treated with either drug. Multiple inflammatory and signaling pathways enriched in cGVHD/BO GCBs were reduced by each drug. GCBs from JQ5- but not JQ1-treated mice were enriched for proproliferative pathways also seen in GCBs from bone marrow-only transplanted mice, likely reflecting their underlying biology in the unperturbed state. In conjunction with in vivo data, these insights led us to conclude that epigenetic targeting of the GC is a viable clinical approach for the treatment of cGVHD, and that the EZH2 inhibitor JQ5 and the BET-bromodomain inhibitor JQ1 demonstrated clinical potential for EZH2i and BETi in patients with cGVHD/BO.
© 2022 by The American Society of Hematology.
Figures



Comment in
-
GVHD…it is all about the microenvironment!Blood. 2022 May 12;139(19):2853-2854. doi: 10.1182/blood.2022015913. Blood. 2022. PMID: 35552645 No abstract available.
References
-
- Greinix HT. Chronic GVHD: progress in salvage treatment? Blood. 2017;130(21):2237-2238. - PubMed
-
- Flowers MED, Parker PM, Johnston LJ, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002;100(2):415-419. - PubMed
-
- Socié G, Stone JV, Wingard JR, et al. ; Late Effects Working Committee of the International Bone Marrow Transplant Registry . Long-term survival and late deaths after allogeneic bone marrow transplantation. N Engl J Med. 1999;341(1):14-21. - PubMed
-
- Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100(2):406-414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

