Colloidal Bismuth Chalcohalide Nanocrystals
- PMID: 35226780
- PMCID: PMC9311208
- DOI: 10.1002/anie.202201747
Colloidal Bismuth Chalcohalide Nanocrystals
Abstract
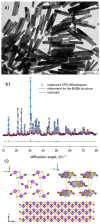
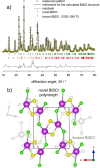
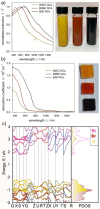
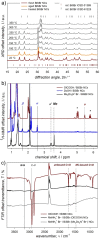
Here we present a colloidal approach to synthesize bismuth chalcohalide nanocrystals (BiEX NCs, in which E=S, Se and X=Cl, Br, I). Our method yields orthorhombic elongated BiEX NCs, with BiSCl crystallizing in a previously unknown polymorph. The BiEX NCs display a composition-dependent band gap spanning the visible spectral range and absorption coefficients exceeding 105 cm-1 . The BiEX NCs show chemical stability at standard laboratory conditions and form colloidal inks in different solvents. These features enable the solution processing of the NCs into robust solid films yielding stable photoelectrochemical current densities under solar-simulated irradiation. Overall, our versatile synthetic protocol may prove valuable in accessing colloidal metal chalcohalide nanomaterials at large and contributes to establish metal chalcohalides as a promising complement to metal chalcogenides and halides for applied nanotechnology.
Keywords: Bismuth Chalcohalides; Colloidal Synthesis; Light-Harvesting; Nanocrystals; Photoelectrochemistry.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
C.Gians., D.Q., S.T., R.G., R.C., A.M., C.Giann., L.M., G.G. are co‐inventors on a patent application entitled “Process for the Production of Nanocrystals of Metal Chalcohalides”, IT 102022000001577.
Figures

References
-
- Meng X., Zhang Z., J. Mol. Catal. A 2016, 423, 533.
-
- Suzuki H., Komatsu N., Ogawa T., Murafuji T., Ikegami T., Matano Y., Organobismuth Chemistry, Elsevier, Amsterdam, 2001.
-
- Mohan R., Nat. Chem. 2010, 2, 336. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

