Diradicals Photogeneration from Chloroaryl-Substituted Carboxylic Acids
- PMID: 35226781
- PMCID: PMC9313617
- DOI: 10.1002/chem.202200313
Diradicals Photogeneration from Chloroaryl-Substituted Carboxylic Acids
Abstract
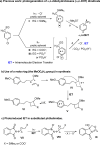
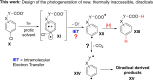
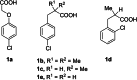
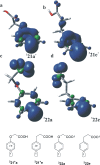
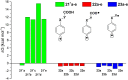
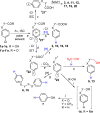
With the aim of generating new, thermally inaccessible diradicals, potentially able to induce a double-strand DNA cleavage, the photochemistry of a set of chloroaryl-substituted carboxylic acids in polar media was investigated. The photoheterolytic cleavage of the Ar-Cl bond occurred in each case to form the corresponding triplet phenyl cations. Under basic conditions, the photorelease of the chloride anion was accompanied by an intramolecular electron-transfer from the carboxylate group to the aromatic radical cationic site to give a diradical species. This latter intermediate could then undergo CO2 loss in a structure-dependent fashion, according to the stability of the resulting diradical, or abstract a hydrogen atom from the medium. In aqueous environment at physiological pH (pH=7.3), both a phenyl cation and a diradical chemistry was observed. The mechanistic scenario and the role of the various intermediates (aryl cations and diradicals) involved in the process was supported by computational analysis.
Keywords: diradicals; heterolytic cleavage; intramolecular electron transfer; phenyl cations; photochemistry.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

