Revisiting the embryogenesis of lip and palate development
- PMID: 35226783
- PMCID: PMC10234451
- DOI: 10.1111/odi.14174
Revisiting the embryogenesis of lip and palate development
Abstract
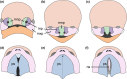
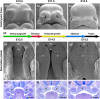
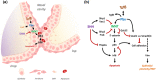
Clefts of the lip and palate (CLP), the major causes of congenital facial malformation globally, result from failure of fusion of the facial processes during embryogenesis. With a prevalence of 1 in 500-2500 live births, CLP causes major morbidity throughout life as a result of problems with facial appearance, feeding, speaking, obstructive apnoea, hearing and social adjustment and requires complex, multi-disciplinary care at considerable cost to healthcare systems worldwide. Long-term outcomes for affected individuals include increased mortality compared with their unaffected siblings. The frequent occurrence and major healthcare burden imposed by CLP highlight the importance of dissecting the molecular mechanisms driving facial development. Identification of the genetic mutations underlying syndromic forms of CLP, where CLP occurs in association with non-cleft clinical features, allied to developmental studies using appropriate animal models is central to our understanding of the molecular events underlying development of the lip and palate and, ultimately, how these are disturbed in CLP.
Keywords: cleft lip; cleft palate; facial development.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

