Entamoeba histolytica Develops Resistance to Complement Deposition and Lysis after Acquisition of Human Complement-Regulatory Proteins through Trogocytosis
- PMID: 35227072
- PMCID: PMC8941920
- DOI: 10.1128/mbio.03163-21
Entamoeba histolytica Develops Resistance to Complement Deposition and Lysis after Acquisition of Human Complement-Regulatory Proteins through Trogocytosis
Abstract
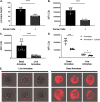
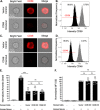
Entamoeba histolytica is the cause of amoebiasis. The trophozoite (amoeba) form of this parasite is capable of invading the intestine and can disseminate through the bloodstream to other organs. The mechanisms that allow amoebae to evade complement deposition during dissemination have not been well characterized. We previously discovered a novel complement-evasion mechanism employed by E. histolytica. E. histolytica ingests small bites of living human cells in a process termed trogocytosis. We demonstrated that amoebae were protected from lysis by human serum following trogocytosis of human cells and that amoebae acquired and displayed human membrane proteins from the cells they ingested. Here, we aimed to define how amoebae are protected from complement lysis after performing trogocytosis. We found that amoebae were protected from complement lysis after ingestion of both human Jurkat T cells and red blood cells and that the level of protection correlated with the amount of material ingested. Trogocytosis of human cells led to a reduction in deposition of C3b on the surface of amoebae. We asked whether display of human complement regulators is involved in amoebic protection, and found that CD59 was displayed by amoebae after trogocytosis. Deletion of a single complement-regulatory protein, CD59 or CD46, from Jurkat cells was not sufficient to alter amoebic protection from lysis, suggesting that multiple, redundant complement regulators mediate amoebic protection. However, exogeneous expression of CD46 or CD55 in amoebae was sufficient to confer protection from lysis. These studies shed light on a novel strategy for immune evasion by a pathogen. IMPORTANCE Entamoeba histolytica is the cause of amoebiasis, a diarrheal disease of global importance. While infection is often asymptomatic, the trophozoite (amoeba) form of this parasite is capable of invading and ulcerating the intestine and can disseminate through the bloodstream to other organs. Understanding how E. histolytica evades the complement system during dissemination is of great interest. Here, we demonstrate for the first time that amoebae that have performed trogocytosis (nibbling of human cells) resist deposition of the complement protein C3b. Amoebae that have performed trogocytosis display the complement-regulatory protein CD59. Overall, our studies suggest that acquisition and display of multiple, redundant complement regulators is involved in amoebic protection from complement lysis. These findings shed light on a novel strategy for immune evasion by a pathogen. Since other parasites use trogocytosis for cell killing, our findings may apply to the pathogenesis of other infections.
Keywords: Entamoeba histolytica; complement; immune evasion; trogocytosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Trogocytosis by Entamoeba histolytica Mediates Acquisition and Display of Human Cell Membrane Proteins and Evasion of Lysis by Human Serum.mBio. 2019 Apr 30;10(2):e00068-19. doi: 10.1128/mBio.00068-19. mBio. 2019. PMID: 31040235 Free PMC article.
-
Cross-species protection suggests Entamoeba histolytica trogocytosis enables complement resistance through the transfer of negative regulators of complement activation.Infect Immun. 2025 Jul 31:e0022025. doi: 10.1128/iai.00220-25. Online ahead of print. Infect Immun. 2025. PMID: 40741974
-
Chew on this: amoebic trogocytosis and host cell killing by Entamoeba histolytica.Trends Parasitol. 2015 Sep;31(9):442-52. doi: 10.1016/j.pt.2015.05.003. Epub 2015 Jun 9. Trends Parasitol. 2015. PMID: 26070402 Free PMC article. Review.
-
Direct and high-throughput assays for human cell killing through trogocytosis by Entamoeba histolytica.Mol Biochem Parasitol. 2020 Sep;239:111301. doi: 10.1016/j.molbiopara.2020.111301. Epub 2020 Jul 17. Mol Biochem Parasitol. 2020. PMID: 32687867 Free PMC article.
-
Taking a bite: Amoebic trogocytosis in Entamoeba histolytica and beyond.Curr Opin Microbiol. 2015 Dec;28:26-35. doi: 10.1016/j.mib.2015.07.009. Epub 2015 Aug 14. Curr Opin Microbiol. 2015. PMID: 26277085 Free PMC article. Review.
Cited by
-
CD55 Facilitates Immune Evasion by Borrelia crocidurae, an Agent of Relapsing Fever.mBio. 2022 Oct 26;13(5):e0116122. doi: 10.1128/mbio.01161-22. Epub 2022 Aug 29. mBio. 2022. PMID: 36036625 Free PMC article.
-
Pilot Study on the Prevalence of Entamoeba gingivalis in Austria-Detection of a New Genetic Variant.Microorganisms. 2023 Apr 22;11(5):1094. doi: 10.3390/microorganisms11051094. Microorganisms. 2023. PMID: 37317068 Free PMC article.
-
Characterization of Entamoeba fatty acid elongases; validation as targets and provision of promising leads for new drugs against amebiasis.PLoS Pathog. 2024 Aug 22;20(8):e1012435. doi: 10.1371/journal.ppat.1012435. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39172749 Free PMC article.
-
Microbial evasion of the complement system: a continuous and evolving story.Front Immunol. 2024 Jan 4;14:1281096. doi: 10.3389/fimmu.2023.1281096. eCollection 2023. Front Immunol. 2024. PMID: 38239357 Free PMC article. Review.
-
Pathogenicity and virulence of Entamoeba histolytica, the agent of amoebiasis.Virulence. 2023 Dec;14(1):2158656. doi: 10.1080/21505594.2022.2158656. Virulence. 2023. PMID: 36519347 Free PMC article. Review.
References
-
- Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, Casey DC, Charlson FJ, Chen AZ, Coates MM, Coggeshall M, Dandona L, Dicker DJ, Erskine HE, Ferrari AJ, Fitzmaurice C, Foreman K, Forouzanfar MH, Fraser MS, Fullman N, Gething PW, Goldberg EM, Graetz N, Haagsma JA, Hay SI, Huynh C, Johnson CO, Kassebaum NJ, Kinfu Y, Kulikoff XR, Kutz M, Kyu HH, Larson HJ, Leung J, Liang X, Lim SS, Lind M, Lozano R, Marquez N, Mensah GA, Mikesell J, Mokdad AH, Mooney MD, Nguyen G, Nsoesie E, Pigott DM, Pinho C, Roth GA, Salomon JA, Sandar L, Silpakit N, et al.. 2016. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1459–1544. doi:10.1016/S0140-6736(16)31012-1. - DOI - PMC - PubMed
-
- Gilchrist CA, Petri SE, Schneider BN, Reichman DJ, Jiang N, Begum S, Watanabe K, Jansen CS, Elliott KP, Burgess SL, Ma JZ, Alam M, Kabir M, Haque R, Petri WA. 2016. Role of the gut microbiota of children in diarrhea due to the protozoan parasite Entamoeba histolytica. J Infect Dis 213:1579–1585. doi:10.1093/infdis/jiv772. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
