Left hemisphere dominance for bilateral kinematic encoding in the human brain
- PMID: 35227374
- PMCID: PMC8887902
- DOI: 10.7554/eLife.69977
Left hemisphere dominance for bilateral kinematic encoding in the human brain
Abstract
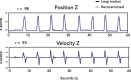
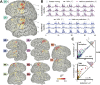
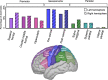
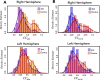
Neurophysiological studies in humans and nonhuman primates have revealed movement representations in both the contralateral and ipsilateral hemispheres. Inspired by clinical observations, we ask if this bilateral representation differs for the left and right hemispheres. Electrocorticography was recorded in human participants during an instructed-delay reaching task, with movements produced with either the contralateral or ipsilateral arm. Using a cross-validated kinematic encoding model, we found stronger bilateral encoding in the left hemisphere, an effect that was present during preparation and was amplified during execution. Consistent with this asymmetry, we also observed better across-arm generalization in the left hemisphere, indicating similar neural representations for right and left arm movements. Notably, these left hemisphere electrodes were centered over premotor and parietal regions. The more extensive bilateral encoding in the left hemisphere adds a new perspective to the pervasive neuropsychological finding that the left hemisphere plays a dominant role in praxis.
Keywords: electrocorticography; human; ipsilateral; lateralization; left hemisphere; motor control; neuroscience; praxis.
Plain language summary
The brain is split into two hemispheres, each playing the leading role in coordinating movement for the opposite side of the body: lesions on the left hemisphere therefore often result in difficulties moving the right arm or leg, and vice versa. In fact, very few anatomical connections exist between a given hemisphere and the body parts on the same (or ‘ipsilateral’) side. Yet, movements produced with only one limb still engage both sides of the brain, with the hemisphere which does not control the action production, still encoding the direction and speed of the movement. Previous evidence also indicate that the two hemispheres may not have equal roles when coordinating ipsilateral movements. Merrick et al. aimed to shed light on these processes; to do so, they measured electrical activity from the surface of the brain of six patients as they moved their arms to reach a screen. The results revealed that, while the right hemisphere only encoded information about the opposite arm, the left hemisphere contained information about both arms. Finer analyses showed that, for both hemispheres, moving the opposite arm was strongly associated with activity in the primary motor cortex, a region which helps to execute movements. However, in the left hemisphere, movements from the ipsilateral arm were related to activity in brain areas involved in planning and integrating different types of sensory information. These findings contribute to a better understanding of how the motor system works, which could ultimately help with the development of brain-machine interfaces for patients who need a neuroprosthetic limb.
© 2022, Merrick et al.
Conflict of interest statement
CM, TD, AB, JL, EC, DK, KL, PW, JC, RT No competing interests declared, RI Senior editor, eLife
Figures



References
-
- Anderson M, Braak CT. Permutation tests for multi-factorial analysis of variance. Journal of Statistical Computation and Simulation. 2003;73:85–113. doi: 10.1080/00949650215733. - DOI
-
- Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using Lme4. arXiv. 2014 https://arxiv.org/abs/1406.5823

