A cellular and molecular atlas reveals the basis of chytrid development
- PMID: 35227375
- PMCID: PMC8887899
- DOI: 10.7554/eLife.73933
A cellular and molecular atlas reveals the basis of chytrid development
Abstract
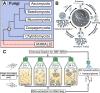
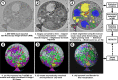
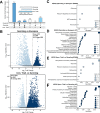
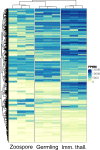
The chytrids (phylum Chytridiomycota) are a major fungal lineage of ecological and evolutionary importance. Despite their importance, many fundamental aspects of chytrid developmental and cell biology remain poorly understood. To address these knowledge gaps, we combined quantitative volume electron microscopy and comparative transcriptome profiling to create an 'atlas' of the cellular and molecular basis of the chytrid life cycle, using the model chytrid Rhizoclosmatium globosum. From our developmental atlas, we describe the transition from the transcriptionally inactive free-swimming zoospore to the more biologically complex germling, and show that lipid processing is multifaceted and dynamic throughout the life cycle. We demonstrate that the chytrid apophysis is a compartmentalised site of high intracellular trafficking, linking the feeding/attaching rhizoids to the reproductive zoosporangium, and constituting division of labour in the chytrid cell plan. We provide evidence that during zoosporogenesis, zoospores display amoeboid morphologies and exhibit endocytotic cargo transport from the interstitial maternal cytoplasm. Taken together, our results reveal insights into chytrid developmental biology and provide a basis for future investigations into non-dikaryan fungal cell biology.
Keywords: Rhizoclosmatium globosum; apophysis; cell biology; chytrid; development; lipid; zoospore.
© 2022, Laundon et al.
Conflict of interest statement
DL, NC, KB, ST, TM, MC No competing interests declared
Figures















References
-
- Barr DJS. Phlyctochytrium arcticum n. sp. (Chytridiales); morphology and physiology. Canadian Journal of Botany. 1970;48:2279–2283. doi: 10.1139/b70-330. - DOI
-
- Barr DJS. Allochytridium expandens rediscovered: morphology, physiology and zoospore ultrastructure. Mycologia. 1986;78:439. doi: 10.2307/3793048. - DOI
-
- Bayne CJ. Phagocytosis and non-self recognition in invertebrates. Bioscience. 1990;40:723–731. doi: 10.2307/1311504. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

