Comparative vaccine effectiveness against severe COVID-19 over time in US hospital administrative data: a case-control study
- PMID: 35227415
- PMCID: PMC8881000
- DOI: 10.1016/S2213-2600(22)00042-X
Comparative vaccine effectiveness against severe COVID-19 over time in US hospital administrative data: a case-control study
Abstract
Background: Research suggests the protection offered by COVID-19 vaccines might wane over time, prompting consideration of booster vaccinations. Data on which vaccines offer the most robust protection over time, and which patients are most vulnerable to attenuating protection, could help inform potential booster programmes. In this study, we used comprehensive hospitalisation data to estimate vaccine effectiveness over time.
Methods: In this case-control study, we used data from a large US health-care system to estimate vaccine effectiveness against severe SARS-CoV-2 infection and examined variation based on time since vaccination, vaccine type, and patients' demographic and clinical characteristics. We compared trends in attenuation of protection across vaccines and used a multivariable model to identify key factors associated with risk for severe breakthrough infection. Patients were considered to have severe COVID-19 if they were admitted to the hospital, had a final coded diagnosis of COVID-19 (according to International Classification of Diseases Tenth Revision code U07.1) or a positive nucleic acid amplification test for symptomatic SARS-CoV-2 during their hospitalisation, and were treated with remdesivir or dexamethasone during hospitalisation.
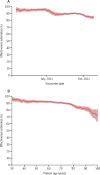
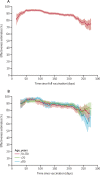
Findings: Between April 1, 2021, and Oct 26, 2021, we observed 9667 admissions for severe COVID-19 (ie, cases). Overall, 1293 (13·4%) of 9667 cases were fully vaccinated at the time of admission, compared with 22 308 (57·7%) of 38 668 controls, who were admitted to hospital for other reasons. The median time between vaccination and hospital admission among cases was 162 days (IQR 118-198). Overall vaccine effectiveness declined mostly over the course of the summer, from 94·5% (95% CI 91·4-96·5) in April, 2021 (pre-delta), to 84·0% (81·6-86·1) by October, 2021. Notably, vaccine effectiveness declined over time, from 94·0% (95% CI 92·8-95·0) at days 50-100 after vaccination to 80·4% (77·8-82·7) by days 200-250 after vaccination. After 250 days, vaccine effectiveness declines were even more notable. Among those who received the BNT162b2 (Pfizer-BioNTech) vaccine, vaccine effectiveness fell from an initial peak of 94·9% (93·2-96·2) to 74·1% (69·6-77·9) by days 200-250 after vaccination. Protection from the mRNA-1273 (Moderna) and Ad26.COV2 (Janssen) vaccines declined less over time, although the latter offered lower overall protection. Holding other factors constant, the risk of severe breakthrough infection was most strongly associated with age older than 80 years (adjusted odds ratio 1·76, 95% CI 1·43-2·15), vaccine type (Pfizer 1·39, 0·98-1·97; Janssen 14·53, 8·43-25·03; both relative to Moderna), time since vaccination (1·05, 1·03-1·07; per week after week 8 when protection peaks, technically), and comorbidities including organ transplantation (3·44, 95% CI 2·12-5·57), cancer (1·93, 1·60-2·33), and immunodeficiency (1·49, 1·13-1·96).
Interpretation: Vaccination remains highly effective against hospitalisation, but vaccine effectiveness declined after 200 days, particularly for older patients or those with specific comorbidities. Additional protection (eg, a booster vaccination) might be warranted for everyone, but especially for these populations. In addition to promoting general vaccine uptake, clinicians and policy makers should consider prioritising booster vaccinations in those most at risk of severe COVID-19.
Funding: None.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

