Hearing Dysfunction After Treatment With Teprotumumab for Thyroid Eye Disease
- PMID: 35227694
- PMCID: PMC9308628
- DOI: 10.1016/j.ajo.2022.02.015
Hearing Dysfunction After Treatment With Teprotumumab for Thyroid Eye Disease
Abstract
Purpose: To characterize the frequency, severity, and resolution of hearing dysfunction in patients treated with teprotumumab for thyroid eye disease (TED).
Design: Prospective observational case series.
Methods: Ophthalmic examination and adverse event assessment, including otologic symptoms, were performed at baseline, after infusions 2, 4, and 8, and at 6-month follow-up in consecutive patients who received at least 4 teprotumumab infusions. Laboratory test results were collected at baseline and during treatment. Audiometry, patulous eustachian tube (PET) testing, and otolaryngology evaluation were obtained for patients with new or worsening otologic symptoms, with a subset obtaining baseline and posttreatment testing.
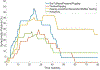
Results: Twenty-seven patients were analyzed (24 females, 3 males, average 56.3 years old). Twenty-two patients (81.5%) developed new subjective otologic symptoms, after a mean of 3.8 infusions (SD 1.8). At 39.2-week average follow-up after the last infusion, most patients with tinnitus (100%), ear plugging/fullness (90.9%), and autophony (83.3%) experienced symptom resolution, whereas only 45.5% (5 of 11) of patients with subjective hearing loss/decreased word comprehension experienced resolution. Six patients underwent baseline and posttreatment audiometry, 5 of whom developed teprotumumab-related sensorineural hearing loss (SNHL) and 1 patient also developed PET. Three of the 5 patients with teprotumumab-related SNHL had persistent subjective hearing loss at last follow-up. A prior history of hearing loss was discovered as a risk factor for teprotumumab-related SNHL (P = .008).
Conclusions: Hearing loss is a concerning adverse event of teprotumumab, and its mechanism and reversibility should be further studied. Until risk factors for hearing loss are better understood, we recommend baseline audiometry with PET testing and repeat testing if new otologic symptoms develop. Screening, monitoring, and prevention guidelines are needed.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Financial Disclosures: Andrea Kossler is a consultant for Horizon Therapeutics and has served on Immunovant advisory boards. Chrysoula Dosiou has served on Horizon Therapeutics advisory boards. Authors Connie Sears, Amee Azad, Brandon Pham, Clara Men, Linus Amarikwa, Daniel Kaplan, Jocelyn Liu, Andrew Hoffman, Austin Swanson, Jennifer Alyono, and Jennifer Lee do not have any conflicts to disclose.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

